Computational Cardiac Modeling Reveals Mechanisms of Ventricular Arrhythmogenesis in Long QT Syndrome Type 8: CACNA1C R858H Mutation Linked to Ventricular Fibrillation
- PMID: 29046645
- PMCID: PMC5632762
- DOI: 10.3389/fphys.2017.00771
Computational Cardiac Modeling Reveals Mechanisms of Ventricular Arrhythmogenesis in Long QT Syndrome Type 8: CACNA1C R858H Mutation Linked to Ventricular Fibrillation
Abstract
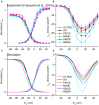
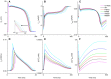
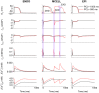
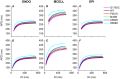
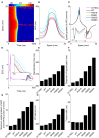
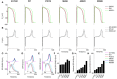
Functional analysis of the L-type calcium channel has shown that the CACNA1C R858H mutation associated with severe QT interval prolongation may lead to ventricular fibrillation (VF). This study investigated multiple potential mechanisms by which the CACNA1C R858H mutation facilitates and perpetuates VF. The Ten Tusscher-Panfilov (TP06) human ventricular cell models incorporating the experimental data on the kinetic properties of L-type calcium channels were integrated into one-dimensional (1D) fiber, 2D sheet, and 3D ventricular models to investigate the pro-arrhythmic effects of CACNA1C mutations by quantifying changes in intracellular calcium handling, action potential profiles, action potential duration restitution (APDR) curves, dispersion of repolarization (DOR), QT interval and spiral wave dynamics. R858H "mutant" L-type calcium current (ICaL ) augmented sarcoplasmic reticulum calcium content, leading to the development of afterdepolarizations at the single cell level and focal activities at the tissue level. It also produced inhomogeneous APD prolongation, causing QT prolongation and repolarization dispersion amplification, rendering R858H "mutant" tissue more vulnerable to the induction of reentry compared with other conditions. In conclusion, altered ICaL due to the CACNA1C R858H mutation increases arrhythmia risk due to afterdepolarizations and increased tissue vulnerability to unidirectional conduction block. However, the observed reentry is not due to afterdepolarizations (not present in our model), but rather to a novel blocking mechanism.
Keywords: CACNA1C mutations; L-type calcium channel; Long QT syndrome; Timothy syndrome; computational cardiac modeling; dispersion of repolarization; ventricular fibrillation.
Figures








Similar articles
-
Long QT syndrome type 8: novel CACNA1C mutations causing QT prolongation and variant phenotypes.Europace. 2014 Dec;16(12):1828-37. doi: 10.1093/europace/euu063. Epub 2014 Apr 12. Europace. 2014. PMID: 24728418
-
Pro-arrhythmogenic effects of CACNA1C G1911R mutation in human ventricular tachycardia: insights from cardiac multi-scale models.Sci Rep. 2016 Aug 9;6:31262. doi: 10.1038/srep31262. Sci Rep. 2016. PMID: 27502440 Free PMC article.
-
Transmural APD heterogeneity determines ventricular arrhythmogenesis in LQT8 syndrome: Insights from Bidomain computational modeling.PLoS One. 2024 Jul 5;19(7):e0305248. doi: 10.1371/journal.pone.0305248. eCollection 2024. PLoS One. 2024. PMID: 38968219 Free PMC article.
-
Transmural dispersion of repolarization and arrhythmogenicity: the Brugada syndrome versus the long QT syndrome.J Electrocardiol. 1999;32 Suppl:158-65. doi: 10.1016/s0022-0736(99)90074-2. J Electrocardiol. 1999. PMID: 10688320 Review.
-
Geno- and phenotypic characteristics and clinical outcomes of CACNA1C gene mutation associated Timothy syndrome, "cardiac only" Timothy syndrome and isolated long QT syndrome 8: A systematic review.Front Cardiovasc Med. 2022 Nov 29;9:1021009. doi: 10.3389/fcvm.2022.1021009. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36523353 Free PMC article.
Cited by
-
Understanding Calmodulin Variants Affecting Calcium-Dependent Inactivation of L-Type Calcium Channels through Whole-Cell Simulation of the Cardiac Ventricular Myocyte.Biomolecules. 2022 Dec 29;13(1):72. doi: 10.3390/biom13010072. Biomolecules. 2022. PMID: 36671457 Free PMC article.
-
PITX2 upregulation increases the risk of chronic atrial fibrillation in a dose-dependent manner by modulating IKs and ICaL -insights from human atrial modelling.Ann Transl Med. 2020 Mar;8(5):191. doi: 10.21037/atm.2020.01.90. Ann Transl Med. 2020. PMID: 32309338 Free PMC article.
-
Unexpected impairment of INa underpins reentrant arrhythmias in a knock-in swine model of Timothy syndrome.Nat Cardiovasc Res. 2023;2(12):1291-1309. doi: 10.1038/s44161-023-00393-w. Epub 2023 Dec 11. Nat Cardiovasc Res. 2023. PMID: 38665938 Free PMC article.
-
Calcium in Brugada Syndrome: Questions for Future Research.Front Physiol. 2018 Aug 10;9:1088. doi: 10.3389/fphys.2018.01088. eCollection 2018. Front Physiol. 2018. PMID: 30147658 Free PMC article. Review.
-
Penetrance and expressivity of the R858H CACNA1C variant in a five-generation pedigree segregating an arrhythmogenic channelopathy.Mol Genet Genomic Med. 2019 Jan;7(1):e00476. doi: 10.1002/mgg3.476. Epub 2018 Oct 21. Mol Genet Genomic Med. 2019. PMID: 30345660 Free PMC article.
References
-
- Adler A., van der Werf C., Postema P. G., Rosso R., Bhuiyan Z. A., Kalman J. M., et al. . (2012). The phenomenon of “QT stunning”: the abnormal QT prolongation provoked by standing persists even as the heart rate returns to normal in patients with long QT syndrome. Heart Rhythm 9, 901–908. 10.1016/j.hrthm.2012.01.026 - DOI - PMC - PubMed
-
- Bai J., Wang K., Zhang H. (2016a). Potential pathogenesis discovery of arrhythmia based on cardiac electrophysiological models: research progress. Prog. Biochem. Biophys. 43, 128–140. 10.16476/j.pibb.2015.0302 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

