TBVAC2020: Advancing Tuberculosis Vaccines from Discovery to Clinical Development
- PMID: 29046674
- PMCID: PMC5632681
- DOI: 10.3389/fimmu.2017.01203
TBVAC2020: Advancing Tuberculosis Vaccines from Discovery to Clinical Development
Abstract
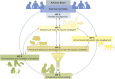
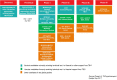
TBVAC2020 is a research project supported by the Horizon 2020 program of the European Commission (EC). It aims at the discovery and development of novel tuberculosis (TB) vaccines from preclinical research projects to early clinical assessment. The project builds on previous collaborations from 1998 onwards funded through the EC framework programs FP5, FP6, and FP7. It has succeeded in attracting new partners from outstanding laboratories from all over the world, now totaling 40 institutions. Next to the development of novel vaccines, TB biomarker development is also considered an important asset to facilitate rational vaccine selection and development. In addition, TBVAC2020 offers portfolio management that provides selection criteria for entry, gating, and priority settings of novel vaccines at an early developmental stage. The TBVAC2020 consortium coordinated by TBVI facilitates collaboration and early data sharing between partners with the common aim of working toward the development of an effective TB vaccine. Close links with funders and other consortia with shared interests further contribute to this goal.
Keywords: bacille Calmette–Guérin; biomarker; clinical trial; discovery; portfolio management; tuberculosis; vaccination.
Figures


References
-
- Calmette A. Sur la vaccination préventive des enfants nouveau-nés contre la tuberculose par le BCG. Ann Institut Pasteur (1927) 41(3):201–32.
-
- WHO. Report on the Global Tuberculosis Epidemic 1998. Geneva: World Health Organization; (1998).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

