Effect of methanol extract of Salviae miltiorrhizae Radix in high-fat diet-induced hyperlipidemic mice
- PMID: 29046711
- PMCID: PMC5640945
- DOI: 10.1186/s13020-017-0150-0
Effect of methanol extract of Salviae miltiorrhizae Radix in high-fat diet-induced hyperlipidemic mice
Abstract
Background: The dried root of Salvia miltiorrhiza, Salviae miltiorrhizae Radix (SR), is one of the most popular medicinal herbs in Asian countries such as China and Korea. In Asian traditional medicine, SR is considered to have a bitter flavor, be slightly cold in nature, and exert therapeutic actions in the heart and liver meridians. Thus, SR has been used to control symptoms related to cardiovascular diseases. Hyperlipidemia is recognized as the main cause of cerebrovascular and heart diseases; consequently, therapeutic strategies for hyperlipidemia have been widely studied. In this study, the effects and molecular targets of methanol extract of SR (SRme) in hyperlipidemic mice were investigated.
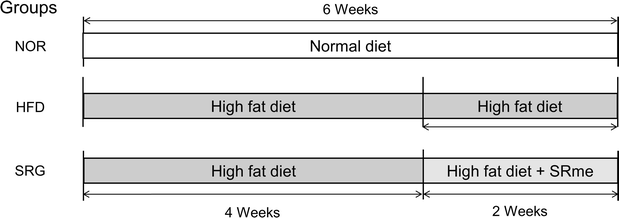
Methods: High-fat diet was fed to mice to induce hyperlipidemia, and measurement of blood cholesterol and triglycerides were conducted to evaluate the effect of SRme on hyperlipidemic mice, and gene expression in mice liver was analyzed to identify key molecules which could be potential targets for developing anti-hyperlipidemic herbal medicines.
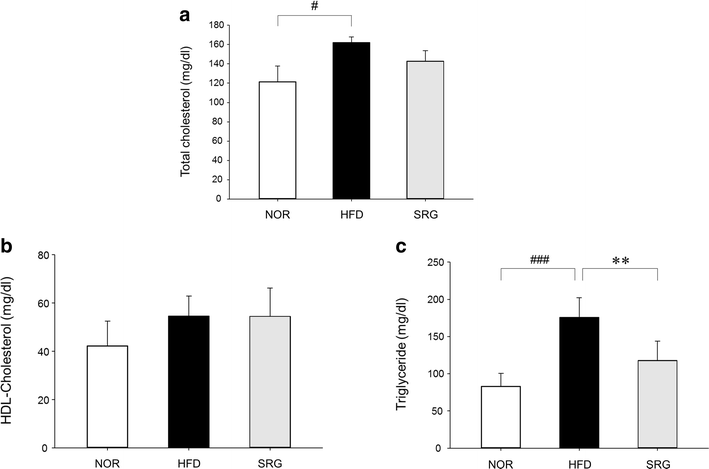
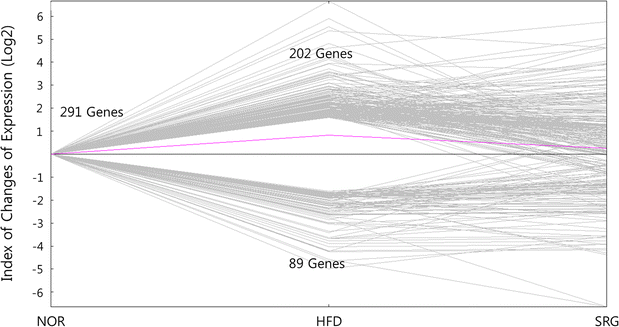
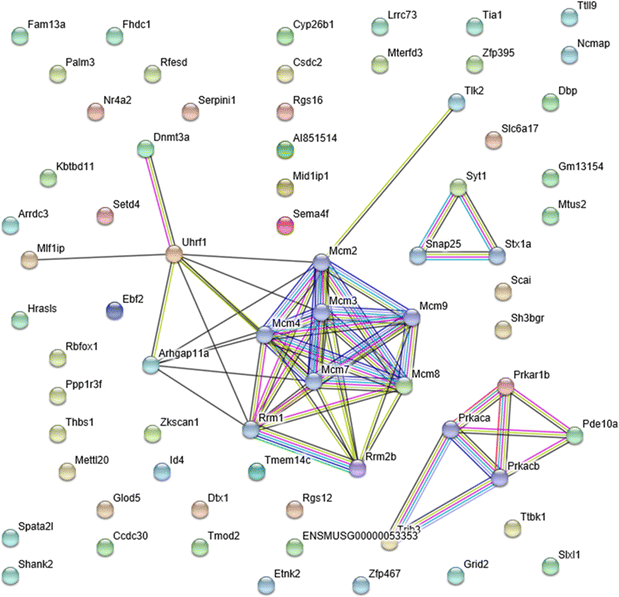
Results: There was no significant effect on the body weight gain of hyperlipidemic mice, but the triglyceride content in blood was significantly reduced by the administration of SRme to hyperlipidemic mice. Proteins such as minichromosome maintenance (Mcm) family which play a key role in DNA replication were identified as molecular targets in the amelioration of hyperlipidemia.
Conclusions: SRme ameliorated hyperlipidemia in high-fat diet fed mice by inhibiting increase of blood serum level of triglycerides. And several proteins such as Mcm proteins were deduced to be molecular targets in treating hyperlipidemia.
Keywords: Cardiovascular diseases; Hyperlipidemia; Salviae miltiorrhizae Radix.
Figures


Similar articles
-
[Study on efficacy markers of Salviae Miltiorrhizae Radix et Rhizoma for promoting blood circulation and remving blood stasis based on systematic traditional Chinese medicine].Zhongguo Zhong Yao Za Zhi. 2020 Jul;45(14):3259-3265. doi: 10.19540/j.cnki.cjcmm.20200210.404. Zhongguo Zhong Yao Za Zhi. 2020. PMID: 32726038 Chinese.
-
[Data mining analysis of regularity of formulas containing Salviae Miltiorrhizae Radix et Rhizoma-Carthami Flos medicin pair in Dictionary of Chinese Medicine Prescription].Zhongguo Zhong Yao Za Zhi. 2016 Feb;41(3):528-531. doi: 10.4268/cjcmm20160328. Zhongguo Zhong Yao Za Zhi. 2016. PMID: 28868875 Chinese.
-
[Fingerprints of Radix Salviae Miltiorrhizae].Zhong Yao Cai. 2001 Jul;24(7):478-80. Zhong Yao Cai. 2001. PMID: 11668736 Chinese.
-
Emerging approaches of traditional Chinese medicine formulas for the treatment of hyperlipidemia.J Ethnopharmacol. 2012 Mar 27;140(2):345-67. doi: 10.1016/j.jep.2012.01.027. Epub 2012 Jan 26. J Ethnopharmacol. 2012. PMID: 22306102 Review.
-
[Research of Chinese medicine pairs (VIII)--Salviae Miltiorrhizae Radix et Rhizoma-Carthami flos].Zhongguo Zhong Yao Za Zhi. 2013 Dec;38(24):4227-31. Zhongguo Zhong Yao Za Zhi. 2013. PMID: 24791521 Review. Chinese.
Cited by
-
Traditional chinese medicine in coronary microvascular disease.Front Pharmacol. 2022 Aug 8;13:929159. doi: 10.3389/fphar.2022.929159. eCollection 2022. Front Pharmacol. 2022. PMID: 36003524 Free PMC article. Review.
-
Yirui Capsules Alleviate Atherosclerosis by Improving the Lipid Profile and Reducing Inflammation in Apolipoprotein E-Deficient Mice.Nutrients. 2018 Jan 29;10(2):142. doi: 10.3390/nu10020142. Nutrients. 2018. PMID: 29382111 Free PMC article.
-
Research Advances in Cardio-Cerebrovascular Diseases of Ligusticum chuanxiong Hort.Front Pharmacol. 2022 Jan 31;12:832673. doi: 10.3389/fphar.2021.832673. eCollection 2021. Front Pharmacol. 2022. PMID: 35173614 Free PMC article. Review.
-
Cardiotonic Pills Plus Recombinant Human Prourokinase Ameliorates Atherosclerotic Lesions in LDLR-/- Mice.Front Physiol. 2019 Sep 10;10:1128. doi: 10.3389/fphys.2019.01128. eCollection 2019. Front Physiol. 2019. PMID: 31551808 Free PMC article.
-
The Relationship between Compound Danshen Dripping Pills with Isosorbide Mononitrate in the Treatment of Elderly Patients with Unstable Angina Pectoris.Evid Based Complement Alternat Med. 2018 Jul 18;2018:3429151. doi: 10.1155/2018/3429151. eCollection 2018. Evid Based Complement Alternat Med. 2018. PMID: 30108652 Free PMC article.
References
-
- Bao CD, Sun B, Lan L, Qiao H, Zhang DF, Liu XY, Wang J, Zhao YS. Interaction between family history of diabetes and hyperlipidemia on risk of diabetes in population with normotension in Harbin: a cross-sectional study. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38(5):611–614. - PubMed
-
- Danese MD, Gleeson M, Griffiths RI, Catterick D, Kutikova L. Methods for estimating costs in patients with hyperlipidemia experiencing their first cardiovascular event in the United Kingdom. J Med Econ. 2017 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

