Single nucleotide polymorphisms and microsatellites in the canine glutathione S-transferase pi 1 (GSTP1) gene promoter
- PMID: 29046813
- PMCID: PMC5635497
- DOI: 10.1186/s40575-017-0050-8
Single nucleotide polymorphisms and microsatellites in the canine glutathione S-transferase pi 1 (GSTP1) gene promoter
Abstract
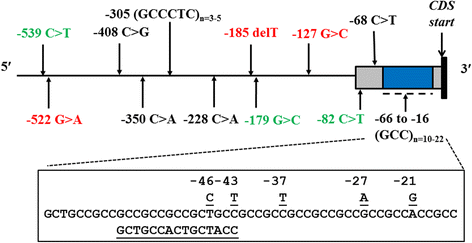
Background: Genetic polymorphisms within the glutathione S-transferase P1 (GSTP1) gene affect the elimination of toxic xenobiotics by the GSTP1 enzyme. In dogs, exposure to environmental chemicals that may be GSTP1 substrates is associated with cancer. The objectives of this study were to investigate the genetic variability in the GSTP1 promoter in a diverse population of 278 purebred dogs, compare the incidence of any variants found between breeds, and predict their effects on gene expression. To provide information on ancestral alleles, a number of wolves, coyotes, and foxes were also sequenced.
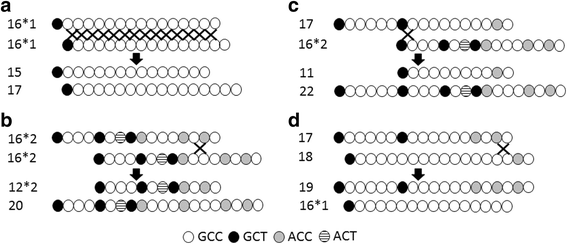
Results: Fifteen single nucleotide polymorphisms (SNPs) and two microsatellites were discovered. Three of these loci were only polymorphic in dogs while three other SNPs were unique to wolves and coyotes. The major allele at c.-46 is T in dogs but is C in the wild canids. The c.-185 delT variant was unique to dogs. The microsatellite located in the 5' untranslated region (5'UTR) was a highly polymorphic GCC tandem repeat, consisting of simple and compound alleles that varied in size from 10 to 22-repeat units. The most common alleles consisted of 11, 16, and 17-repeats. The 11-repeat allele was found in 10% of dogs but not in the other canids. Unequal recombination and replication slippage between similar and distinct alleles may be the mechanism for the multiple microsatellites observed. Twenty-eight haplotypes were constructed in the dog, and an additional 8 were observed in wolves and coyotes. While the most common haplotype acrossbreeds was the wild-type *1A(17), other prevalent haplotypes included *3A(11) in Greyhounds, *6A(16) in Labrador Retrievers, *9A(16) in Golden Retrievers, and *8A(19) in Standard Poodles. Boxers and Siberian Huskies exhibited minimal haplotypic diversity. Compared to the simple 16*1 allele, the compound 16*2 allele (found in 12% of dogs) may interfere with transcription factor binding and/or the stability of the GSTP1 transcript.
Conclusions: Dogs and other canids exhibit extensive variation in the GSTP1 promoter. Genetic polymorphisms within distinct haplotypes prevalent in certain breeds can affect GSTP1 expression and carcinogen detoxification, and thus may be useful as genetic markers for cancer in dogs.
Keywords: Breed; Canine; Carcinogen; GSTP1; Microsatellite; Polymorphism; Promoter.
Conflict of interest statement
Ethics approval and consent to participate
The Drake IACUC reviewed the study protocol and the committee determined that these experiments did not require IACUC approval. The IACUC decision is reproduced below: −.
Date: February 9, 2017.
From: Kimberly Huey, Ph.D. IACUC Chair.
To: James Sacco, Ph.D.
Re: Sequencing of the Glutathione-S-Transferase P-1 (GSTP1) gene promoter in canids.
The IACUC has reviewed your protocol “Sequencing of the Glutathione-S-Transferase P-1 (GSTP1) gene promoter in canids” and the committee has determined that these experiments do not require IACUC approval. This research is considered exempt from IACUC approval based upon the following stipulations:
1. Veterinarians or animal handlers will be collecting all the saliva samples and will provide these samples to your laboratory. No one in your laboratory will have any physical contact with the wolves.
2. No samples will be collected on the Drake University campus and no wolves will be housed in Drake University animal facilities.
If you subsequently plan to make any significant changes regarding the use of animals in this protocol you must inform the IACUC before continuing the research.
Thank you for your cooperation and good luck with your research.
Kimberly Huey, PhD.
Associate Professor of Physiology.
Chair, IACUC.
Drake University.
271–4853.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Resequencing of the TMF-1 (TATA Element Modulatory Factor) regulated protein (TRNP1) gene in domestic and wild canids.Canine Med Genet. 2023 Nov 15;10(1):10. doi: 10.1186/s40575-023-00133-0. Canine Med Genet. 2023. PMID: 37968761 Free PMC article.
-
A simple repeat polymorphism in the MITF-M promoter is a key regulator of white spotting in dogs.PLoS One. 2014 Aug 12;9(8):e104363. doi: 10.1371/journal.pone.0104363. eCollection 2014. PLoS One. 2014. PMID: 25116146 Free PMC article.
-
Polymorphisms in the canine monoamine oxidase a (MAOA) gene: identification and variation among five broad dog breed groups.Canine Genet Epidemiol. 2017 Jan 13;4:1. doi: 10.1186/s40575-016-0040-2. eCollection 2017. Canine Genet Epidemiol. 2017. PMID: 28101368 Free PMC article.
-
Utility of canine microsatellites in revealing the relationships of pure bred dogs.J Hered. 1999 Jan-Feb;90(1):104-7. doi: 10.1093/jhered/90.1.104. J Hered. 1999. PMID: 9987914
-
Glutathione S-transferase P1, maternal smoking, and asthma in children: a haplotype-based analysis.Environ Health Perspect. 2008 Mar;116(3):409-15. doi: 10.1289/ehp.10655. Environ Health Perspect. 2008. PMID: 18335111 Free PMC article.
Cited by
-
Genetic and environmental risk for lymphoma in boxer dogs.J Vet Intern Med. 2020 Sep;34(5):2068-2077. doi: 10.1111/jvim.15849. Epub 2020 Jul 15. J Vet Intern Med. 2020. PMID: 32667715 Free PMC article.
References
-
- Sherratt PJ, Pulford DJ, Harrison DJ, Green T, Hayes JD. Evidence that human class Theta glutathione S-transferase T1-1 can catalyse the activation of dichloromethane, a liver and lung carcinogen in the mouse. Comparison of the tissue distribution of GST T1-1 with that of classes Alpha, Mu and Pi GST in human. Biochem J. 1997;326:837–846. doi: 10.1042/bj3260837. - DOI - PMC - PubMed
-
- Berhane K, Widersten M, Engstrom A, Kozarich JW, Mannervik B. Detoxication of base propenals and other alpha, beta-unsaturated aldehyde products of radical reactions and lipid peroxidation by human glutathione transferases. Proc Natl Acad Sci U S A. 1994;91:1480–1484. doi: 10.1073/pnas.91.4.1480. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

