Self-assembly of chiral fluorescent nanoparticles based on water-soluble L-tryptophan derivatives of p-tert- butylthiacalix[4]arene
- PMID: 29046831
- PMCID: PMC5629409
- DOI: 10.3762/bjnano.8.184
Self-assembly of chiral fluorescent nanoparticles based on water-soluble L-tryptophan derivatives of p-tert- butylthiacalix[4]arene
Abstract
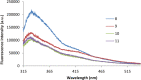
New water-soluble tetra-substituted derivatives of p-tert-butylthiacalix[4]arene containing fragments of L-tryptophan in cone and 1,3-alternate conformations were obtained. It was shown that the resulting compounds form stable, positively charged aggregates of 86-134 nm in diameter in water at a concentration of 1 × 10-4 M as confirmed by dynamic light scattering, scanning electron microscopy and transmission electron microscopy. It was established that these aggregates are fluorescently active and chiral. A distinctive feature of the compounds is the pronounced dependence of their spectral (emission and chiroptical) properties on the polarity of the solvent and the length of the linker between the macrocyclic and fluorophore parts of the molecule.
Keywords: fluorescence; nanoparticles; self-assembly; thiacalixarene; tryptophan.
Figures





Similar articles
-
Decasubstituted Pillar[5]arene Derivatives Containing L-Tryptophan and L-Phenylalanine Residues: Non-Covalent Binding and Release of Fluorescein from Nanoparticles.Int J Mol Sci. 2023 Apr 22;24(9):7700. doi: 10.3390/ijms24097700. Int J Mol Sci. 2023. PMID: 37175406 Free PMC article.
-
Synthesis of Water-Soluble Amino Functionalized Multithiacalix[4]arene via Quaternization of Tertiary Amino Groups.Molecules. 2018 May 8;23(5):1117. doi: 10.3390/molecules23051117. Molecules. 2018. PMID: 29738518 Free PMC article.
-
Albumin/Thiacalix[4]arene Nanoparticles as Potential Therapeutic Systems: Role of the Macrocycle for Stabilization of Monomeric Protein and Self-Assembly with Ciprofloxacin.Int J Mol Sci. 2022 Sep 2;23(17):10040. doi: 10.3390/ijms231710040. Int J Mol Sci. 2022. PMID: 36077448 Free PMC article.
-
Structure-Activity Relationship of the Thiacalix[4]arenes Family with Sulfobetaine Fragments: Self-Assembly and Cytotoxic Effect against Cancer Cell Lines.Molecules. 2022 Feb 17;27(4):1364. doi: 10.3390/molecules27041364. Molecules. 2022. PMID: 35209152 Free PMC article.
-
The synthesis of new amphiphilic p-tert-butylthiacalix[4]arenes containing peptide fragments and their interaction with DNA.Org Biomol Chem. 2015 Jun 7;13(21):5894-904. doi: 10.1039/c5ob00548e. Org Biomol Chem. 2015. PMID: 25921225
Cited by
-
PAMAM-Calix-Dendrimers: Second Generation Synthesis, Fluorescent Properties and Catecholamines Binding.Pharmaceutics. 2022 Dec 8;14(12):2748. doi: 10.3390/pharmaceutics14122748. Pharmaceutics. 2022. PMID: 36559243 Free PMC article.
-
Decasubstituted Pillar[5]arene Derivatives Containing L-Tryptophan and L-Phenylalanine Residues: Non-Covalent Binding and Release of Fluorescein from Nanoparticles.Int J Mol Sci. 2023 Apr 22;24(9):7700. doi: 10.3390/ijms24097700. Int J Mol Sci. 2023. PMID: 37175406 Free PMC article.
-
Doxorubicin aqueous systems at low concentrations: Interconnection between self-organization, fluorescent and physicochemical properties, and action on hydrobionts.Front Chem. 2022 Dec 1;10:1063278. doi: 10.3389/fchem.2022.1063278. eCollection 2022. Front Chem. 2022. PMID: 36531320 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
