Optical techniques for cervical neoplasia detection
- PMID: 29046833
- PMCID: PMC5629403
- DOI: 10.3762/bjnano.8.186
Optical techniques for cervical neoplasia detection
Abstract
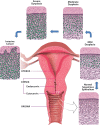
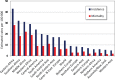
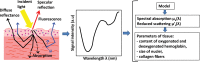
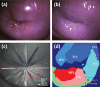
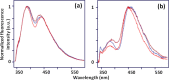
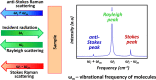
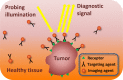
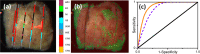
This paper provides an overview of the current research in the field of optical techniques for cervical neoplasia detection and covers a wide range of the existing and emerging technologies. Using colposcopy, a visual inspection of the uterine cervix with a colposcope (a binocular microscope with 3- to 15-fold magnification), has proven to be an efficient approach for the detection of invasive cancer. Nevertheless, the development of a reliable and cost-effective technique for the identification of precancerous lesions, confined to the epithelium (cervical intraepithelial neoplasia) still remains a challenging problem. It is known that even at early stages the neoplastic transformations of cervical tissue induce complex changes and modify both structural and biochemical properties of tissues. The different methods, including spectroscopic (diffuse reflectance spectroscopy, induced fluorescence and autofluorescence spectroscopy, Raman spectroscopy) and imaging techniques (confocal microscopy, optical coherence tomography, Mueller matrix imaging polarimetry, photoacoustic imaging), probe different tissue properties that may serve as optical biomarkers for diagnosis. Both the advantages and drawbacks of these techniques for the diagnosis of cervical precancerous lesions are discussed and compared.
Keywords: Mueller polarimetry; Raman spectroscopy; cervical intraepithelial neoplasia; confocal endomicroscopy; nanotheranostics; optical coherence tomography; optical spectroscopy.
Figures




Similar articles
-
Confocal endomicroscopy: a novel imaging technique for in vivo histology of cervical intraepithelial neoplasia.Expert Rev Med Devices. 2007 Nov;4(6):863-71. doi: 10.1586/17434440.4.6.863. Expert Rev Med Devices. 2007. PMID: 18035951 Review.
-
Characterization of cervical tissue using Mueller matrix polarimetry.Lasers Med Sci. 2023 Jan 20;38(1):46. doi: 10.1007/s10103-023-03712-6. Lasers Med Sci. 2023. PMID: 36662327 Review.
-
Optical imaging of the cervix.Cancer. 2003 Nov 1;98(9 Suppl):2015-27. doi: 10.1002/cncr.11678. Cancer. 2003. PMID: 14603538 Review.
-
Emerging enhanced imaging technologies of the esophagus: spectroscopy, confocal laser endomicroscopy, and optical coherence tomography.J Surg Res. 2015 May 15;195(2):502-14. doi: 10.1016/j.jss.2015.02.045. Epub 2015 Feb 21. J Surg Res. 2015. PMID: 25819772 Review.
-
Endoscopic imaging: emerging optical techniques for the detection of colorectal neoplasia.Curr Opin Gastroenterol. 2008 Jan;24(1):64-9. doi: 10.1097/MOG.0b013e3282f2df8d. Curr Opin Gastroenterol. 2008. PMID: 18043235 Review.
Cited by
-
Cervical Imaging in the Low Resource Setting: A Review.Biosensors (Basel). 2022 Sep 24;12(10):786. doi: 10.3390/bios12100786. Biosensors (Basel). 2022. PMID: 36290924 Free PMC article. Review.
-
A Framework for Cervical Cancer Elimination in Low-and-Middle-Income Countries: A Scoping Review and Roadmap for Interventions and Research Priorities.Front Public Health. 2021 Jul 1;9:670032. doi: 10.3389/fpubh.2021.670032. eCollection 2021. Front Public Health. 2021. PMID: 34277540 Free PMC article.
-
Polarimetric measurement utility for pre-cancer detection from uterine cervix specimens.Biomed Opt Express. 2018 Oct 25;9(11):5691-5702. doi: 10.1364/BOE.9.005691. eCollection 2018 Nov 1. Biomed Opt Express. 2018. PMID: 30460156 Free PMC article.
-
Phototheranostics of Cervical Neoplasms with Chlorin e6 Photosensitizer.Cancers (Basel). 2022 Jan 2;14(1):211. doi: 10.3390/cancers14010211. Cancers (Basel). 2022. PMID: 35008375 Free PMC article.
-
Lectins in Cervical Screening.Cancers (Basel). 2020 Jul 16;12(7):1928. doi: 10.3390/cancers12071928. Cancers (Basel). 2020. PMID: 32708812 Free PMC article.
References
-
- World Health Organization . World Cancer Report. Geneva, Switzerland: World Health Organization; 2014. Chapter 5.12; pp. 465–481.
-
- WHO [Aug 8;2017 ];Cervical cancer. Available from: http://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer...
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
