Application of visible-light photosensitization to form alkyl-radical-derived thin films on gold
- PMID: 29046834
- PMCID: PMC5629420
- DOI: 10.3762/bjnano.8.187
Application of visible-light photosensitization to form alkyl-radical-derived thin films on gold
Abstract
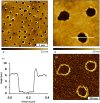
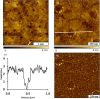
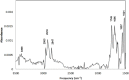
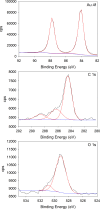
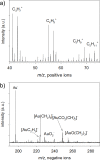
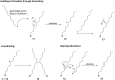
Visible-light irradiation of phthalimide esters in the presence of the photosensitizer [Ru(bpy)3]2+ and the stoichiometric reducing agent benzyl nicotinamide results in the formation of alkyl radicals under mild conditions. This approach to radical generation has proven useful for the synthesis of small organic molecules. Herein, we demonstrate for the first time the visible-light photosensitized deposition of robust alkyl thin films on Au surfaces using phthalimide esters as the alkyl radical precursors. In particular, we combine visible-light photosensitization with particle lithography to produce nanostructured thin films, the thickness of which can be measured easily using AFM cursor profiles. Analysis with AFM demonstrated that the films are robust and resistant to mechanical force while contact angle goniometry suggests a multilayered and disordered film structure. Analysis with IRRAS, XPS, and TOF SIMS provides further insights.
Keywords: TOF SIMS; atomic force microscopy; organic thin film; particle lithography; photosensitization.
Figures






Similar articles
-
Application of visible light photocatalysis with particle lithography to generate polynitrophenylene nanostructures.J Am Chem Soc. 2014 Oct 15;136(41):14438-44. doi: 10.1021/ja505521k. Epub 2014 Oct 6. J Am Chem Soc. 2014. PMID: 25244537
-
One-step formation of bifunctionnal aryl/alkyl grafted films on conducting surfaces by the reduction of diazonium salts in the presence of alkyl iodides.Langmuir. 2015 May 19;31(19):5406-15. doi: 10.1021/acs.langmuir.5b00754. Epub 2015 May 4. Langmuir. 2015. PMID: 25893643
-
Nanoscale Studies of Organic Radicals: Surface, Interface, and Spinterface.Acc Chem Res. 2018 Mar 20;51(3):753-760. doi: 10.1021/acs.accounts.7b00612. Epub 2018 Feb 21. Acc Chem Res. 2018. PMID: 29465979
-
Alkanephosphonates on hafnium-modified gold: a new class of self-assembled organic monolayers.J Am Chem Soc. 2007 Mar 14;129(10):2803-7. doi: 10.1021/ja065598a. Epub 2007 Feb 14. J Am Chem Soc. 2007. PMID: 17298060
-
Nanoclusters in polymer matrices prepared by co-deposition from a gas phase.Adv Colloid Interface Sci. 2005 Nov 30;116(1-3):263-76. doi: 10.1016/j.cis.2005.04.005. Epub 2005 Oct 19. Adv Colloid Interface Sci. 2005. PMID: 16242110 Review.
References
-
- Combellas C, Kanoufi F, Osman Z, Pinson J, Adenier A, Hallais F. Electrochim Acta. 2011;56:1476–1484. doi: 10.1016/j.electacta.2010.10.062. - DOI
-
- Berisha A, Combellas C, Hallais F, Kanoufi F, Pinson J, Podvorica F I. Chem Mater. 2011;23:3449–3459. doi: 10.1021/cm200579b. - DOI
-
- Chehimi M M, Hallais G, Matrab T, Pinson J, Podvorica F I. J Phys Chem C. 2008;112:18559–18565. doi: 10.1021/jp807044j. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
