A systematic study of the controlled generation of crystalline iron oxide nanoparticles on graphene using a chemical etching process
- PMID: 29046849
- PMCID: PMC5629405
- DOI: 10.3762/bjnano.8.202
A systematic study of the controlled generation of crystalline iron oxide nanoparticles on graphene using a chemical etching process
Abstract
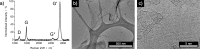
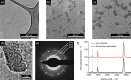
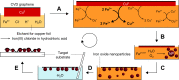
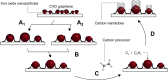
Chemical vapor deposition (CVD) of carbon precursors employing a metal catalyst is a well-established method for synthesizing high-quality single-layer graphene. Yet the main challenge of the CVD process is the required transfer of a graphene layer from the substrate surface onto a chosen target substrate. This process is delicate and can severely degrade the quality of the transferred graphene. The protective polymer coatings typically used generate residues and contamination on the ultrathin graphene layer. In this work, we have developed a graphene transfer process which works without a coating and allows the transfer of graphene onto arbitrary substrates without the need for any additional post-processing. During the course of our transfer studies, we found that the etching process that is usually employed can lead to contamination of the graphene layer with the Faradaic etchant component FeCl3, resulting in the deposition of iron oxide Fe x O y nanoparticles on the graphene surface. We systematically analyzed the removal of the copper substrate layer and verified that crystalline iron oxide nanoparticles could be generated in controllable density on the graphene surface when this process is optimized. It was further confirmed that the Fe x O y particles on graphene are active in the catalytic growth of carbon nanotubes when employing a water-assisted CVD process.
Keywords: carbon nanotubes; chemical vapor deposition; graphene; iron oxide; nanoparticles.
Figures




Similar articles
-
Designed CVD growth of graphene via process engineering.Acc Chem Res. 2013 Oct 15;46(10):2263-74. doi: 10.1021/ar400057n. Acc Chem Res. 2013. PMID: 23869401
-
The CVD graphene transfer procedure introduces metallic impurities which alter the graphene electrochemical properties.Nanoscale. 2014 Jan 7;6(1):472-6. doi: 10.1039/c3nr05230c. Epub 2013 Nov 11. Nanoscale. 2014. PMID: 24217345
-
Copper-vapor-assisted chemical vapor deposition for high-quality and metal-free single-layer graphene on amorphous SiO2 substrate.ACS Nano. 2013 Aug 27;7(8):6575-82. doi: 10.1021/nn402847w. Epub 2013 Jul 24. ACS Nano. 2013. PMID: 23869700
-
Controllable Growth of Graphene on Liquid Surfaces.Adv Mater. 2019 Mar;31(9):e1800690. doi: 10.1002/adma.201800690. Epub 2018 Dec 9. Adv Mater. 2019. PMID: 30536644 Review.
-
Art etching of graphene.Nanoscale Horiz. 2024 Jul 22;9(8):1230-1249. doi: 10.1039/d4nh00077c. Nanoscale Horiz. 2024. PMID: 38958571 Review.
References
-
- Boehm H P, Clauss A, Fischer G O, Hofmann U. Z Anorg Allg Chem. 1962;316:119–127. doi: 10.1002/zaac.19623160303. - DOI
-
- Boehm H P, Clauss A, Fischer G O, Hofmann U. Z Naturforsch, B. 1962;17:150.
-
- Fitzer E, Köchling K-H, Boehm H P, Marsh H. Pure Appl Chem. 1995;67:473. doi: 10.1351/pac199567030473. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
