Synthesis of 4 H-Benzo[ e][ 1,3]oxazin-4-ones by a Carbonylation-Cyclization Domino Reaction of ortho-Halophenols and Cyanamide
- PMID: 29046856
- PMCID: PMC5641915
- DOI: 10.1002/open.201700130
Synthesis of 4 H-Benzo[ e][ 1,3]oxazin-4-ones by a Carbonylation-Cyclization Domino Reaction of ortho-Halophenols and Cyanamide
Abstract
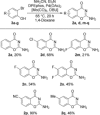
A mild and convenient one-step preparation of 4H-1,3-benzoxazin-4-ones by a domino carbonylation-cyclization process is developed. Readily available ortho-iodophenols are subjected to palladium-catalyzed carbonylative coupling with Mo(CO)6 and cyanamide, followed by a spontaneous, intramolecular cyclization to afford 4H-1,3-benzoxazin-4-ones in moderate to excellent yields. Furthermore, the scope of the reaction is extended to include challenging ortho-bromophenols. Finally, to highlight the versatility of the developed method, Mo(CO)6 is successfully replaced with a wide array of CO-releasing reagents, such as oxalyl chloride, phenyl formate, 9-methylfluorene-9-carbonyl chloride, and formic acid, making this an appealing strategy for the synthesis of 4H-benzo[e][1,3]oxazin-4-ones.
Keywords: 4H-benzo[e][1,3]oxazin-4-ones; carbonylation; domino reactions; halophenols; heterocycles.
Figures



Similar articles
-
Synthesis of 4H-Benzo[e][1,3]oxazin-4-ones by a Carbonylation-Cyclization Domino Reaction of ortho-Halophenols and Cyanamide.ChemistryOpen. 2017 Sep 7;6(5):609. doi: 10.1002/open.201700145. eCollection 2017 Oct. ChemistryOpen. 2017. PMID: 29046853 Free PMC article.
-
Pd-Catalyzed Carbonylative Synthesis of 4H-Benzo[d][1,3]Oxazin-4-Ones Using Benzene-1,3,5-Triyl Triformate as the CO Source.Chemistry. 2021 Nov 22;27(65):16219-16224. doi: 10.1002/chem.202103137. Epub 2021 Oct 4. Chemistry. 2021. PMID: 34529291
-
Synthesis of 2-Aminoquinazolinones via Carbonylative Coupling of ortho-Iodoanilines and Cyanamide.J Org Chem. 2016 Apr 1;81(7):2966-73. doi: 10.1021/acs.joc.6b00249. Epub 2016 Mar 22. J Org Chem. 2016. PMID: 26967689
-
Palladium-Catalyzed Carbonylative Sonogashira Transformations: Advancements and Insights.Chem Rec. 2025 May;25(5):e202400251. doi: 10.1002/tcr.202400251. Epub 2025 Mar 27. Chem Rec. 2025. PMID: 40145855 Review.
-
Palladium-Catalyzed Carbonylative Multicomponent Reactions.Chemistry. 2017 Mar 2;23(13):2973-2987. doi: 10.1002/chem.201603623. Epub 2016 Oct 31. Chemistry. 2017. PMID: 27797399 Review.
Cited by
-
Palladium-Catalyzed Carbonylative Cyclization of 1-Alkynyl-2-iodo-d-glucal.Org Lett. 2024 Oct 11;26(40):8621-8625. doi: 10.1021/acs.orglett.4c03337. Epub 2024 Sep 30. Org Lett. 2024. PMID: 39348604 Free PMC article.
-
Recent Progress in the Synthesis of Benzoxazin-4-Ones, Applications in N-Directed Ortho-Functionalizations, and Biological Significance.Molecules. 2024 Dec 3;29(23):5710. doi: 10.3390/molecules29235710. Molecules. 2024. PMID: 39683871 Free PMC article. Review.
References
-
- Vitaku E., Smith D. T., Njardarson J. T., J. Med. Chem. 2014, 57, 10257–10274. - PubMed
-
- Alper-Hayta S., Aki-Sener E., Tekiner-Gulbas B., Yildiz I., Temiz-Arpaci O., Yalcin I., Altanlar N., Eur. J. Med. Chem. 2006, 41, 1398–1404. - PubMed
-
- Morrison R., Al-Rawi J. M. A., Jennings I. G., Thompson P. E., Angove M. J., Eur. J. Med. Chem. 2016, 110, 326–339. - PubMed
-
- Ihmaid S. K., Al-Rawi J. M. A., Bradley C. J., Angove M. J., Robertson M. N., Eur. J. Med. Chem. 2012, 57, 85–101. - PubMed
-
- Morrison R., Zheng Z., Jennings I. G., Thompson P. E., Al-Rawi J. M. A., Bioorg. Med. Chem. Lett. 2016, 26, 5534–5538. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

