Embryonic Stem Cell-Like Subpopulations in Venous Malformation
- PMID: 29046873
- PMCID: PMC5632722
- DOI: 10.3389/fmed.2017.00162
Embryonic Stem Cell-Like Subpopulations in Venous Malformation
Abstract
Background: Venous malformation (VM) consists of a network of ectatic anomalous thin-walled venous channels. A role for an activating TIE2 mutation in the development of the dilated luminal vessels in VM, and its proposed involvement of embryonic stem cells (ESCs), led us to investigate the expression of ESC markers in subcutaneous VM (SCVM) and intramuscular VM (IMVM).
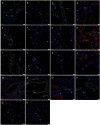
Methods: Formalin-fixed paraffin-embedded sections of SCVM from seven patients and IMVM samples from seven patients were analyzed for the expression of Nanog, pSTAT3, OCT4, SOX2, SALL4, and CD44, using 3,3'-diaminobenzidine (DAB) immunohistochemical (IHC) staining. All these samples did not express lymphatic marker D2-40. NanoString mRNA analysis and RT-PCR were performed on snap-frozen samples of SCVM (n = 3) and IMVM (n = 3) from the respective original cohorts of patients included in DAB IHC staining. To confirm co-expression of two proteins, immunofluorescent (IF) IHC staining on two representative samples of IMVM and SCVM samples from the original cohorts of patients included for DAB IHC staining was performed.
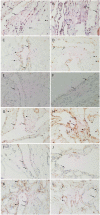
Results: DAB IHC staining demonstrated expression of all of the above ESC markers in both SCVM and IMVM samples. IF IHC staining showed that these markers were localized to the endothelium within these lesions and that Nanog, pSTAT3, SOX2, and CD44 were also expressed by cells outside of the endothelium. NanoString mRNA analysis confirmed transcription activation of pSTAT3, OCT4, and CD44. RT-qPCR confirmed transcription activation of Nanog, SOX2, and SALL4.
Conclusion: Our findings support the presence of two ESC-like subpopulations, one within and one outside of the endothelium, of both SCVM and IMVM. Given that the endothelial ESC-like subpopulation expresses the more primitive marker, OCT4, it is exciting to speculate that they give rise to the non-endothelial subpopulation.
Keywords: embryonic; markers; pathogenesis; stem cells; venous malformation.
Figures


Similar articles
-
Embryonic Stem Cell-like Population within Venous Malformation Expresses the Renin-Angiotensin System.Plast Reconstr Surg Glob Open. 2019 Apr 2;7(4):e2170. doi: 10.1097/GOX.0000000000002170. eCollection 2019 Apr. Plast Reconstr Surg Glob Open. 2019. PMID: 31321175 Free PMC article.
-
Characterization of Cancer Stem Cells in Moderately Differentiated Buccal Mucosal Squamous Cell Carcinoma.Front Surg. 2016 Aug 2;3:46. doi: 10.3389/fsurg.2016.00046. eCollection 2016. Front Surg. 2016. PMID: 27532037 Free PMC article.
-
The Identification of Three Cancer Stem Cell Subpopulations within Moderately Differentiated Lip Squamous Cell Carcinoma.Front Surg. 2017 Mar 6;4:12. doi: 10.3389/fsurg.2017.00012. eCollection 2017. Front Surg. 2017. PMID: 28321397 Free PMC article.
-
Review of the endothelial pathogenic mechanism of TIE2-related venous malformation.J Vasc Surg Venous Lymphat Disord. 2017 Sep;5(5):740-748. doi: 10.1016/j.jvsv.2017.05.001. Epub 2017 May 16. J Vasc Surg Venous Lymphat Disord. 2017. PMID: 28818232 Review.
-
A comparative study of quantitative immunohistochemistry and quantum dot immunohistochemistry for mutation carrier identification in Lynch syndrome.J Clin Pathol. 2011 Mar;64(3):208-14. doi: 10.1136/jcp.2010.084418. Epub 2010 Dec 22. J Clin Pathol. 2011. PMID: 21177748 Review.
Cited by
-
Expression of Cathepsins B, D, and G in Extracranial Arterio-Venous Malformation.Front Surg. 2021 Aug 2;8:676871. doi: 10.3389/fsurg.2021.676871. eCollection 2021. Front Surg. 2021. PMID: 34409065 Free PMC article.
-
Regression of a venous malformation during angiotensin-converting enzyme inhibitor treatment for hypertension.J Vasc Surg Cases Innov Tech. 2022 Sep 17;8(4):657-659. doi: 10.1016/j.jvscit.2022.09.004. eCollection 2022 Dec. J Vasc Surg Cases Innov Tech. 2022. PMID: 36262918 Free PMC article.
-
Role of cereblon in angiogenesis and in mediating the antiangiogenic activity of immunomodulatory drugs.FASEB J. 2020 Sep;34(9):11395-11404. doi: 10.1096/fj.201903060RR. Epub 2020 Jul 17. FASEB J. 2020. PMID: 32677118 Free PMC article.
-
Cell Populations Expressing Stemness-Associated Markers in Vascular Anomalies.Front Surg. 2021 Feb 9;7:610758. doi: 10.3389/fsurg.2020.610758. eCollection 2020. Front Surg. 2021. PMID: 33634164 Free PMC article. Review.
-
Methylome analysis of endothelial cells suggests new insights on sporadic brain arteriovenous malformation.Heliyon. 2024 Jul 30;10(15):e35126. doi: 10.1016/j.heliyon.2024.e35126. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170526 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

