Fatty Acid-Mimetic Micelles for Dual Delivery of Antigens and Imidazoquinoline Adjuvants
- PMID: 29046894
- PMCID: PMC5642296
- DOI: 10.1021/acsbiomaterials.6b00408
Fatty Acid-Mimetic Micelles for Dual Delivery of Antigens and Imidazoquinoline Adjuvants
Abstract
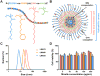
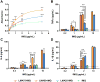
Vaccine design has undergone a shift towards the use of purified protein subunit vaccines, which offer increased safety and greater control over antigen specificity, but at the expense of immunogenicity. Here we report the development of a new polymer-based vaccine delivery platform engineered to enhance immunity through the co-delivery of protein antigens and the Toll-like receptor 7 (TLR7) agonist imiquimod (IMQ). Owing to the preferential solubility of IMQ in fatty acids, a series of block copolymer micelles with a fatty acid-mimetic core comprising lauryl methacrylate (LMA) and methacrylic acid (MAA), and a poly(ethylene glycol) methyl ether methacrylate (PEGMA) corona decorated with pyridyl disulfide ethyl methacrylate (PDSM) moieties for antigen conjugation were synthesized via reversible addition-fragmentation chain transfer (RAFT) polymerization. Carriers composed of 50 mole% LMA (LMA50) demonstrated the highest IMQ loading (2.2 w/w%) and significantly enhanced the immunostimulatory capacity of IMQ to induce dendritic cell maturation and proinflammatory cytokine production. Conjugation of a model antigen, ovalbumin (OVA), to the corona of IMQ-loaded LMA50 micelles enhanced in vitro antigen uptake and cross-presentation on MHC class I (MHC-I). A single intranasal (IN) immunization of mice with carriers co-loaded with IMQ and OVA elicited significantly higher pulmonary and systemic CD8+ T cell responses and increased serum IgG titer relative to a soluble formulation of antigen and adjuvant. Collectively, these data demonstrate that rationally designed fatty acid-mimetic micelles enhance intracellular antigen and IMQ delivery and have potential as synthetic vectors for enhancing the immunogenicity of subunit vaccines.
Keywords: Imiquimod; RAFT polymerization; Toll-like receptor 7; block copolymer micelles; mucosal immunity; subunit vaccine.
Figures
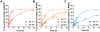

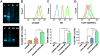
Similar articles
-
Neutral polymer micelle carriers with pH-responsive, endosome-releasing activity modulate antigen trafficking to enhance CD8(+) T cell responses.J Control Release. 2014 Oct 10;191:24-33. doi: 10.1016/j.jconrel.2014.03.041. Epub 2014 Mar 31. J Control Release. 2014. PMID: 24698946 Free PMC article.
-
pH-Responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides.ACS Nano. 2013 May 28;7(5):3912-25. doi: 10.1021/nn305466z. Epub 2013 Apr 30. ACS Nano. 2013. PMID: 23590591 Free PMC article.
-
Dual-Responsive Glycopolymers for Intracellular Codelivery of Antigen and Lipophilic Adjuvants.Mol Pharm. 2022 Dec 5;19(12):4705-4716. doi: 10.1021/acs.molpharmaceut.2c00750. Epub 2022 Nov 14. Mol Pharm. 2022. PMID: 36374992 Free PMC article.
-
Particulate formulations for the delivery of poly(I:C) as vaccine adjuvant.Adv Drug Deliv Rev. 2013 Oct;65(10):1386-99. doi: 10.1016/j.addr.2013.05.013. Epub 2013 Jun 7. Adv Drug Deliv Rev. 2013. PMID: 23751781 Review.
-
Bioresponsive functional nanogels as an emerging platform for cancer therapy.Expert Opin Drug Deliv. 2018 Jul;15(7):703-716. doi: 10.1080/17425247.2018.1497607. Epub 2018 Jul 16. Expert Opin Drug Deliv. 2018. PMID: 29976103 Review.
Cited by
-
Engineering self-assembled materials to study and direct immune function.Adv Drug Deliv Rev. 2017 May 15;114:60-78. doi: 10.1016/j.addr.2017.03.005. Epub 2017 Apr 6. Adv Drug Deliv Rev. 2017. PMID: 28392305 Free PMC article. Review.
-
Engineering endosomolytic nanocarriers of diverse morphologies using confined impingement jet mixing.Nanoscale. 2023 Oct 12;15(39):16016-16029. doi: 10.1039/d3nr02874g. Nanoscale. 2023. PMID: 37753868 Free PMC article.
-
Bionanotechnology for vaccine design.Curr Opin Biotechnol. 2018 Aug;52:80-88. doi: 10.1016/j.copbio.2018.03.003. Epub 2018 Mar 26. Curr Opin Biotechnol. 2018. PMID: 29597075 Free PMC article. Review.
-
Biomaterial strategies for generating therapeutic immune responses.Adv Drug Deliv Rev. 2017 May 15;114:3-18. doi: 10.1016/j.addr.2017.04.009. Epub 2017 Apr 25. Adv Drug Deliv Rev. 2017. PMID: 28455189 Free PMC article. Review.
-
Huang Lian Jie Du Decoction enhances the anti-tumor efficacy of immune checkpoint inhibitors by activating TLR7/8 signalling in melanoma.BMC Complement Med Ther. 2024 Apr 11;24(1):156. doi: 10.1186/s12906-024-04444-y. BMC Complement Med Ther. 2024. PMID: 38605368 Free PMC article.
References
-
- Coffman RL, Sher A, Seder RA. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity. 2010;33(4):492–503. http://dx.doi.org/10.1016/j.immuni.2010.10.002. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
