ROS Signaling in the Pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS)
- PMID: 29047084
- PMCID: PMC7120947
- DOI: 10.1007/978-3-319-63245-2_8
ROS Signaling in the Pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS)
Abstract
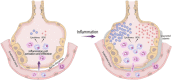
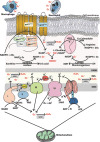
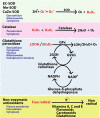
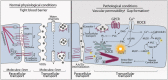
The generation of reactive oxygen species (ROS) plays an important role for the maintenance of cellular processes and functions in the body. However, the excessive generation of oxygen radicals under pathological conditions such as acute lung injury (ALI) and its most severe form acute respiratory distress syndrome (ARDS) leads to increased endothelial permeability. Within this hallmark of ALI and ARDS, vascular microvessels lose their junctional integrity and show increased myosin contractions that promote the migration of polymorphonuclear leukocytes (PMNs) and the transition of solutes and fluids in the alveolar lumen. These processes all have a redox component, and this chapter focuses on the role played by ROS during the development of ALI/ARDS. We discuss the origins of ROS within the cell, cellular defense mechanisms against oxidative damage, the role of ROS in the development of endothelial permeability, and potential therapies targeted at oxidative stress.
Keywords: Catalase; Cytochrome P450; Glutathione; Lung injury; Mitochondrial respiratory chain; NADPH oxidase; Nitric oxide synthase; Polymorphonuclear leukocytes; Pulmonary endothelial cell; Reactive oxygen species; Superoxide dismutase; Xanthine oxidase.
Figures

Similar articles
-
Endothelial pathomechanisms in acute lung injury.Vascul Pharmacol. 2008 Oct-Dec;49(4-6):119-33. doi: 10.1016/j.vph.2008.06.009. Epub 2008 Jul 29. Vascul Pharmacol. 2008. PMID: 18722553 Free PMC article. Review.
-
Neutrophils in acute lung injury.Front Biosci (Landmark Ed). 2012 Jun 1;17(6):2278-83. doi: 10.2741/4051. Front Biosci (Landmark Ed). 2012. PMID: 22652778 Review.
-
Transcription factor NF-E2-related factor 2 plays a critical role in acute lung injury/acute respiratory distress syndrome (ALI/ARDS) by regulating ferroptosis.PeerJ. 2024 Jul 30;12:e17692. doi: 10.7717/peerj.17692. eCollection 2024. PeerJ. 2024. PMID: 39670103 Free PMC article. Review.
-
ROS-activated calcium signaling mechanisms regulating endothelial barrier function.Cell Calcium. 2016 Sep;60(3):163-71. doi: 10.1016/j.ceca.2016.02.002. Epub 2016 Feb 17. Cell Calcium. 2016. PMID: 26905827 Free PMC article. Review.
-
Targeting NOX4 alleviates sepsis-induced acute lung injury via attenuation of redox-sensitive activation of CaMKII/ERK1/2/MLCK and endothelial cell barrier dysfunction.Redox Biol. 2020 Sep;36:101638. doi: 10.1016/j.redox.2020.101638. Epub 2020 Jul 13. Redox Biol. 2020. PMID: 32863203 Free PMC article.
Cited by
-
Vimentin regulation of autophagy activation in lung fibroblasts in response to lipopolysaccharide exposure in vitro.Ann Transl Med. 2021 Feb;9(4):304. doi: 10.21037/atm-20-5129. Ann Transl Med. 2021. PMID: 33708931 Free PMC article.
-
Implications of SARS-Cov-2 infection on eNOS and iNOS activity: Consequences for the respiratory and vascular systems.Nitric Oxide. 2021 Jun 1;111-112:64-71. doi: 10.1016/j.niox.2021.04.003. Epub 2021 Apr 6. Nitric Oxide. 2021. PMID: 33831567 Free PMC article. Review.
-
Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression.Adv Biol Regul. 2020 Aug;77:100741. doi: 10.1016/j.jbior.2020.100741. Epub 2020 Jul 4. Adv Biol Regul. 2020. PMID: 32773102 Free PMC article. Review.
-
Pharmacologic therapies of ARDS: From natural herb to nanomedicine.Front Pharmacol. 2022 Oct 28;13:930593. doi: 10.3389/fphar.2022.930593. eCollection 2022. Front Pharmacol. 2022. PMID: 36386221 Free PMC article. Review.
-
Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms.Pharmacol Res. 2021 Jan;163:105224. doi: 10.1016/j.phrs.2020.105224. Epub 2020 Sep 29. Pharmacol Res. 2021. PMID: 33007416 Free PMC article. Review.
References
-
- Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: The berlin definition. Journal of the American Medical Association. 2012;307(23):2526–2533. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

