Mitochondrial and Metabolic Drivers of Pulmonary Vascular Endothelial Dysfunction in Pulmonary Hypertension
- PMID: 29047100
- PMCID: PMC5810356
- DOI: 10.1007/978-3-319-63245-2_24
Mitochondrial and Metabolic Drivers of Pulmonary Vascular Endothelial Dysfunction in Pulmonary Hypertension
Abstract
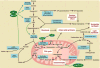
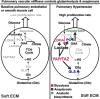
Pulmonary hypertension (PH) is a deadly and increasingly prevalent vascular disease characterized by excessive pulmonary vascular remodeling and right ventricular dysfunction which leads to right heart failure, multiorgan dysfunction, and premature death. The cause of the vascular remodeling in PH remains elusive, and thus current treatments are marginally effective and prognosis of PH remains poor. Increasing evidence indicates the pathogenic importance of endothelial dysfunction in PH. However, the underlying mechanisms of such dysfunction are not well described. Endothelial apoptosis and hyperproliferation have been identified in patients with PH. Both are linked with the increased oxidative stress and inflammatory responses, and are influenced by various genetic and exogenous stresses. Importantly, contrary to historic dogma that suggested a negligible role for mitochondria and energy balance in endothelial pathology, recent findings have implicated the role of endothelial metabolism directly in PH. This chapter addresses the emerging role of mitochondria in pulmonary vascular endothelial dysfunction in PH. A more sophisticated understanding of the biochemical, metabolic, molecular, and physiologic underpinnings of this emerging paradigm should enable the development of a new generation of targeted therapies that will stunt or reverse pulmonary vascular remodeling.
Keywords: Endothelial cell; Metabolism; Mitochondria; Pulmonary hypertension.
Figures

Similar articles
-
Sildenafil preserves lung endothelial function and prevents pulmonary vascular remodeling in a rat model of diastolic heart failure.Circ Heart Fail. 2011 Mar;4(2):198-206. doi: 10.1161/CIRCHEARTFAILURE.110.957050. Epub 2011 Jan 7. Circ Heart Fail. 2011. PMID: 21216837
-
Metabolic Reprogramming and Redox Signaling in Pulmonary Hypertension.Adv Exp Med Biol. 2017;967:241-260. doi: 10.1007/978-3-319-63245-2_14. Adv Exp Med Biol. 2017. PMID: 29047090
-
Metabolic dysfunction in pulmonary hypertension: from basic science to clinical practice.Eur Respir Rev. 2017 Dec 20;26(146):170094. doi: 10.1183/16000617.0094-2017. Print 2017 Dec 31. Eur Respir Rev. 2017. PMID: 29263174 Free PMC article. Review.
-
Endothelial cell energy metabolism, proliferation, and apoptosis in pulmonary hypertension.Compr Physiol. 2011 Jan;1(1):357-72. doi: 10.1002/cphy.c090005. Compr Physiol. 2011. PMID: 23737177 Free PMC article. Review.
-
Metabolic reprogramming, oxidative stress, and pulmonary hypertension.Redox Biol. 2023 Aug;64:102797. doi: 10.1016/j.redox.2023.102797. Epub 2023 Jun 24. Redox Biol. 2023. PMID: 37392518 Free PMC article. Review.
Cited by
-
CircGSAP regulates the cell cycle of pulmonary microvascular endothelial cells via the miR-942-5p sponge in pulmonary hypertension.Front Cell Dev Biol. 2022 Aug 11;10:967708. doi: 10.3389/fcell.2022.967708. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36060794 Free PMC article.
-
Computational repurposing of therapeutic small molecules from cancer to pulmonary hypertension.Sci Adv. 2021 Oct 22;7(43):eabh3794. doi: 10.1126/sciadv.abh3794. Epub 2021 Oct 20. Sci Adv. 2021. PMID: 34669463 Free PMC article.
-
Metabolic Reprogramming of Vascular Endothelial Cells: Basic Research and Clinical Applications.Front Cell Dev Biol. 2021 Feb 18;9:626047. doi: 10.3389/fcell.2021.626047. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33681205 Free PMC article. Review.
-
Molecular basis of the association between transcription regulators nuclear respiratory factor 1 and inhibitor of DNA binding protein 3 and the development of microvascular lesions.Microvasc Res. 2022 May;141:104337. doi: 10.1016/j.mvr.2022.104337. Epub 2022 Feb 7. Microvasc Res. 2022. PMID: 35143811 Free PMC article. Review.
-
TRPM2 channel-mediated cell death: An important mechanism linking oxidative stress-inducing pathological factors to associated pathological conditions.Redox Biol. 2020 Oct;37:101755. doi: 10.1016/j.redox.2020.101755. Epub 2020 Oct 16. Redox Biol. 2020. PMID: 33130440 Free PMC article. Review.
References
-
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–887. - PubMed
-
- Simonneau G, et al. Updated clinical classification of pulmonary hypertension. Journal of the American College of Cardiology. 2013;62(25 Suppl):D34–D41. - PubMed
-
- Lee R, Channon KM, Antoniades C. Therapeutic strategies targeting endothelial function in humans: Clinical implications. Current Vascular Pharmacology. 2012;10(1):77–93. - PubMed
-
- Michelakis ED. Spatio-temporal diversity of apoptosis within the vascular wall in pulmonary arterial hypertension: Heterogeneous BMP signaling may have therapeutic implications. Circulation Research. 2006;98(2):172–175. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

