Recurrent moderate hypoglycemia exacerbates oxidative damage and neuronal death leading to cognitive dysfunction after the hypoglycemic coma
- PMID: 29047291
- PMCID: PMC6501509
- DOI: 10.1177/0271678X17733640
Recurrent moderate hypoglycemia exacerbates oxidative damage and neuronal death leading to cognitive dysfunction after the hypoglycemic coma
Abstract
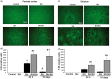
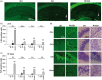
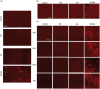
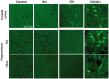
Moderate recurrent hypoglycemia (RH) is frequent in Type 1 diabetes mellitus (TIDM) patients who are under intensive insulin therapy increasing the risk for severe hypoglycemia (SH). The consequences of RH are not well understood and its repercussions on neuronal damage and cognitive function after a subsequent episode of SH have been poorly investigated. In the current study, we have addressed this question and observed that previous RH during seven consecutive days exacerbated oxidative damage and neuronal death induced by a subsequent episode of SH accompanied by a short period of coma, in the parietal cortex, the striatum and mainly in the hippocampus. These changes correlated with a severe decrease in reduced glutathione content (GSH), and a significant spatial and contextual memory deficit. Administration of the antioxidant, N-acetyl-L-cysteine, (NAC) reduced neuronal death and prevented cognitive impairment. These results demonstrate that previous RH enhances brain vulnerability to acute hypoglycemia and suggests that this effect is mediated by the decline in the antioxidant defense and oxidative damage. The present results highlight the importance of an adequate control of moderate hypoglycemic episodes in TIDM.
Keywords: Diabetes; antioxidants; cognitive impairment/decline; glucose; hippocampus; hypoglycemia; risk factors; selective neuronal death.
Figures



References
-
- Donelly LA, Morris AD, Frier BM, et al. Frequency and predictors of hypoglycaemia in Type 1 and insulin-treated type 2 diabetes: a population-based study. Diab Med 2005; 22: 749–755. - PubMed
-
- Heller S, Colagiuri S, Vaaler S, et al. Hypoglycaemia with insulin aspart: a doubleblind randomised, crossover trial in subjects with Type 1 diabetes. Diab Med 2004; 21: 769–775. - PubMed
-
- Blasetti A, Di Giulio C, Tocco AM, et al. Variables associated with severe hypoglycemia in children and adolescents with Type 1 diabetes: a population-based study. Pediatr Diab 2011; 12: 4–10. - PubMed
-
- Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol 2014; 10: 711–722. - PubMed
-
- Languren G, Montiel T, Julio-Amilpas A, et al. Neuronal damage and cognitive impairment associated with hypoglycemia: an integrated view. Neurochem Int 2013; 63: 331–343. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

