Comparison and predictors of treatment adherence and remission among patients with schizophrenia treated with paliperidone palmitate or atypical oral antipsychotics in community behavioral health organizations
- PMID: 29047368
- PMCID: PMC5648472
- DOI: 10.1186/s12888-017-1507-8
Comparison and predictors of treatment adherence and remission among patients with schizophrenia treated with paliperidone palmitate or atypical oral antipsychotics in community behavioral health organizations
Abstract
Background: Nonadherence to antipsychotic treatment increases the likelihood of relapse and progressive symptomatology in patients with schizophrenia. Atypical long-acting injectables, including paliperidone palmitate (PP), may increase adherence and improve symptoms. This study compared and assessed predictors of treatment patterns and symptom remission among schizophrenia patients treated with PP versus atypical oral antipsychotic therapy (OAT) in community behavioral health organizations (CBHOs).
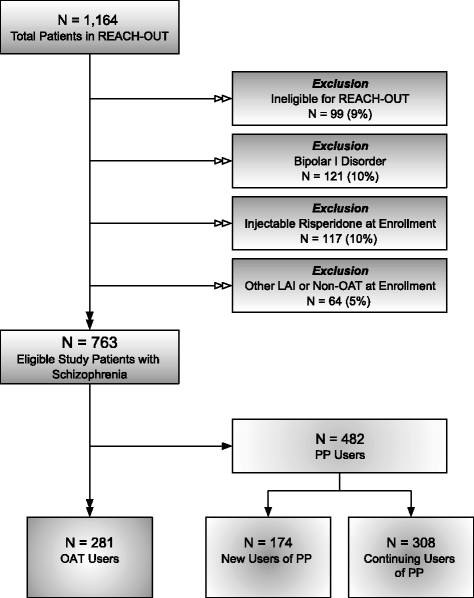
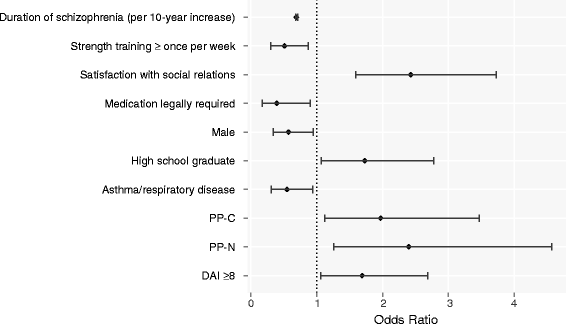
Methods: This retrospective cohort analysis evaluated 763 patients with schizophrenia and new (PP-N; N = 174) or continuing (PP-C; N = 308) users of PP, or new users of OAT (N = 281) at enrollment in the REACH-OUT study (2010-2013). Treatment outcomes assessed at 1 year were discontinuation, and adherence, measured by proportion of days covered (PDC) or medication possession ratio (MPR). Remission status was assessed using the Structured Clinical Interview for Symptoms of Remission (SCI-SR). A machine learning platform, Reverse Engineering and Forward Simulation (REFS™), was used to identify predictors of study outcomes. Multivariate Cox and generalized linear regressions estimated the adjusted hazard ratios (HRs) or odds ratios (ORs) with 95% confidence intervals.
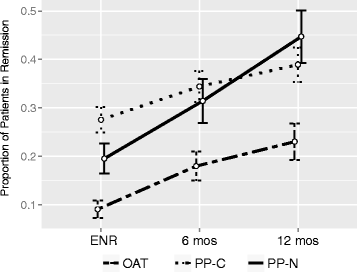
Results: Among PP-N users, 27% discontinued their initial treatment regimen versus 51% (p < 0.001) of OAT users. PP-N (vs OAT; HR = 0.49 [0.31-0.76]) users and males (HR = 0.65 [0.46-0.92]) had significantly lower rates of discontinuation. Relative to OAT, PP-N had a 36% [31%-42%] higher MPR and a 10-fold increased achievement of PDC ≥80% (OR = 10.46 [5.72-19.76]). PP users were significantly more likely to achieve remission in follow-up (PP-N vs OAT: OR = 2.65 [1.39-5.05]; PP-C vs OAT: OR = 1.83 [1.03-3.25]).
Conclusions: Relative to OAT, PP was associated with improved adherence, less frequent treatment discontinuation, and improved symptom remission in this CBHO study population.
Keywords: Adherence; Community behavioral health organization; Oral antipsychotics; Paliperidone palmitate; Remission; Schizophrenia.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the New England Institutional Review Board, and conducted in accordance with the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from all subjects prior to study enrollment.
Consent for publication
Not applicable.
Competing interests
All authors were employed by GNS Healthcare or Janssen Scientific Affairs, LLC, at the time this research was conducted. KJ and CB are Johnson & Johnson stockholders. REFS™ is proprietary to GNS Healthcare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- National Institute of Mental Health. Schizophrenia. http://www.nimh.nih.gov/health/topics/schizophrenia/index.shtml. Last revised November 2016. Accessed 9 Nov 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

