Mildly elevated lactate levels are associated with microcirculatory flow abnormalities and increased mortality: a microSOAP post hoc analysis
- PMID: 29047411
- PMCID: PMC5646128
- DOI: 10.1186/s13054-017-1842-7
Mildly elevated lactate levels are associated with microcirculatory flow abnormalities and increased mortality: a microSOAP post hoc analysis
Abstract
Background: Mildly elevated lactate levels (i.e., 1-2 mmol/L) are increasingly recognized as a prognostic finding in critically ill patients. One of several possible underlying mechanisms, microcirculatory dysfunction, can be assessed at the bedside using sublingual direct in vivo microscopy. We aimed to evaluate the association between relative hyperlactatemia, microcirculatory flow, and outcome.
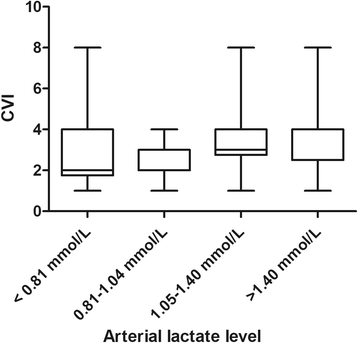
Methods: This study was a predefined subanalysis of a multicenter international point prevalence study on microcirculatory flow abnormalities, the Microcirculatory Shock Occurrence in Acutely ill Patients (microSOAP). Microcirculatory flow abnormalities were assessed with sidestream dark-field imaging. Abnormal microcirculatory flow was defined as a microvascular flow index (MFI) < 2.6. MFI is a semiquantitative score ranging from 0 (no flow) to 3 (continuous flow). Associations between microcirculatory flow abnormalities, single-spot lactate measurements, and outcome were analyzed.
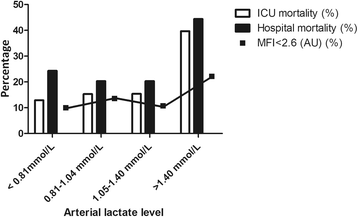
Results: In 338 of 501 patients, lactate levels were available. For this substudy, all 257 patients with lactate levels ≤ 2 mmol/L (median [IQR] 1.04 [0.80-1.40] mmol/L) were included. Crude ICU mortality increased with each lactate quartile. In a multivariable analysis, a lactate level > 1.5 mmol/L was independently associated with a MFI < 2.6 (OR 2.5, 95% CI 1.1-5.7, P = 0.027).
Conclusions: In a heterogeneous ICU population, a single-spot mildly elevated lactate level (even within the reference range) was independently associated with increased mortality and microvascular flow abnormalities. In vivo microscopy of the microcirculation may be helpful in discriminating between flow- and non-flow-related causes of mildly elevated lactate levels.
Trial registration: ClinicalTrials.gov, NCT01179243 . Registered on August 3, 2010.
Keywords: Intensive care; Lactate; Microcirculation; Prevalence; SDF imaging.
Conflict of interest statement
Ethics approval and consent to participate
All centers obtained medical ethics approval via their local ethics committees. Written informed consent (or a waiver, if applicable) was obtained in accordance with local applicable law.
Consent for publication
Not applicable.
Competing interests
The institution of NARV and ECB has received grant support from a local hospital fund (Medical Center Leeuwarden, unrestricted grant). NIS has served as a board member for the Cumberland Data and Safety Monitoring Board. The institution of NIS has received grant support from Biosite, Cheetah Medical, Rapid Pathogen Screening, and Thermo Fisher Scientific. RMP has consulted for Massimo and Edwards Lifesciences and has lectured for Nestle Health Sciences. The institution of RMP has received grant support from Nestle Health Sciences, LiDCO, and Cephalon (some are equipment loans, not funding). DP has consulted for Vygon Italia. RLM has consulted for AbbVie, CSL Behring, AM-Pharma, Grifols, Ardea Biosciences, and GlaxoSmithKline; has provided expert testimony for Nell DyMott; has lectured for AbbVie; and has stock options with Astute Medical. The institution of RLM has received grant support from Spectral Medical, AlloCure, and Eli Lilly. AHR has served as a board member for MSD; has consulted and lectured for Pfizer, Astellas Pharma, Novartis, and Brahms; and has received support for travel from Pfizer, MSD, Astellas Pharma, Novartis, and Brahms. HSC has disclosed government work. The institution of JD has lectured for LFB Biopharmaceuticals. SHu is employed by Royal Devon and Exeter Hospital NHS Trust (intensive care consultant). CJ reports receiving (all outside the submitted work) grants and personal fees from Actelion Pharmaceuticals, Bayer Healthcare, and ZOLL Medical, as well as personal fees from Boehringer Ingelheim, Vifor Pharma, Pfizer, Abbott Vascular, Boston Scientific, and Novartis. CS has disclosed government work. CI has developed SDF imaging and is listed as inventor on related patents commercialized by MicroVision Medical (MVM) under a license from the Academic Medical Center, Amsterdam, The Netherlands. CI has been a consultant for MVM in the past but has not been involved with this company for more than 5 years, except for still holding shares. Braedius Medical, a company owned by a relative of CI, has developed and designed a handheld microscope called CytoCam-IDF imaging. CI has no financial relationship with Braedius Medical of any sort (i.e., has never owned shares or received consultancy or speaker fees from Braedius Medical).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Jansen TC, van Bommel J, Woodward R, Mulder PGH, Bakker J. Association between blood lactate levels, Sequential Organ Failure Assessment subscores, and 28-day mortality during early and late intensive care unit stay: a retrospective observational study. Crit Care Med. 2009;37:2369–74. doi: 10.1097/CCM.0b013e3181a0f919. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

