Salicylic acid-dependent immunity contributes to resistance against Rhizoctonia solani, a necrotrophic fungal agent of sheath blight, in rice and Brachypodium distachyon
- PMID: 29048113
- PMCID: PMC5765516
- DOI: 10.1111/nph.14849
Salicylic acid-dependent immunity contributes to resistance against Rhizoctonia solani, a necrotrophic fungal agent of sheath blight, in rice and Brachypodium distachyon
Abstract
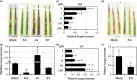
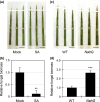
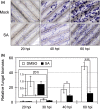
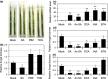
Rhizoctonia solani is a soil-borne fungus causing sheath blight. In consistent with its necrotrophic life style, no rice cultivars fully resistant to R. solani are known, and agrochemical plant defense activators used for rice blast, which upregulate a phytohormonal salicylic acid (SA)-dependent pathway, are ineffective towards this pathogen. As a result of the unavailability of genetics, the infection process of R. solani remains unclear. We used the model monocotyledonous plants Brachypodium distachyon and rice, and evaluated the effects of phytohormone-induced resistance to R. solani by pharmacological, genetic and microscopic approaches to understand fungal pathogenicity. Pretreatment with SA, but not with plant defense activators used in agriculture, can unexpectedly induce sheath blight resistance in plants. SA treatment inhibits the advancement of R. solani to the point in the infection process in which fungal biomass shows remarkable expansion and specific infection machinery is developed. The involvement of SA in R. solani resistance is demonstrated by SA-deficient NahG transgenic rice and the sheath blight-resistant B. distachyon accessions, Bd3-1 and Gaz-4, which activate SA-dependent signaling on inoculation. Our findings suggest a hemi-biotrophic nature of R. solani, which can be targeted by SA-dependent plant immunity. Furthermore, B. distachyon provides a genetic resource that can confer disease resistance against R. solani to plants.
Keywords: Brachypodium distachyon; Rhizoctonia solani; biotroph; disease resistance; necrotroph; rice; salicylic acid (SA); sheath blight.
© 2017 The Authors. New Phytologist © 2017 New Phytologist Trust.
Figures



References
-
- Anderson J, Hane J, Stoll T, Pain N, Hastie M, Kaur P, Hoogland C, Gorman J, Singh K. 2016. Proteomic analysis of Rhizoctonia solani identifies infection‐specific, redox associated proteins and insight into adaptation to different plant hosts. Molecular & Cellular Proteomics 15: 1188–1203. - PMC - PubMed
-
- Anderson N. 1982. The genetics and pathology of Rhizoctonia solani . Annual Review of Phytopathology 20: 329–347.
-
- Blanco F, Salinas P, Cecchini N, Jordana X, Van Hummelen P, Alvarez M, Holuigue L. 2009. Early genomic responses to salicylic acid in Arabidopsis. Plant Molecular Biology 70: 79–102. - PubMed
-
- Brooks S. 2007. Sensitivity to a phytotoxin from Rhizoctonia solani correlates with sheath blight susceptibility in rice. Phytopathology 97: 1207–1212. - PubMed
-
- Budge G, Shaw M, Colyer A, Pietravalle S, Boonham N. 2009. Molecular tools to investigate Rhizoctonia solani distribution in soil. Plant Pathology 58: 1071–1080.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

