Development of a Modular Synthetic Route to (+)-Pleuromutilin, (+)-12-epi-Mutilins, and Related Structures
- PMID: 29048164
- PMCID: PMC7024634
- DOI: 10.1021/jacs.7b09869
Development of a Modular Synthetic Route to (+)-Pleuromutilin, (+)-12-epi-Mutilins, and Related Structures
Abstract
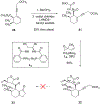
We describe the development of an enantioselective synthetic route to (+)-pleuromutilin (1), (+)-12-epi-mutilin, and related derivatives. A key hydrindanone was prepared in three steps and 48% overall yield from cyclohex-2-en-1-one. 1,4-Hydrocyanation provided a nitrile (53%, or 85% based on recovered starting material) that was converted to the eneimide 57 in 80% yield by the 1,2-addition of methyllithium to the nitrile function, cyclization, and in situ acylation with di-tert-butyldicarbonate. The eneimide 57 was employed in a 2-fold neopentylic coupling reaction with an organolithium reagent derived from the alkyl iodides (R)- or (S)-30, which contain the C11-C13 atoms of the target, to provide diastereomeric diketones in 60% or 48% yield (for coupling with (R)- or (S)-30, respectively). The diketone derived from (S)-30 contains the (S)-C12 stereochemistry found in pleuromutilin and was elaborated to an alkynylaldehyde. Nickel-catalyzed reductive cyclization of this alkynylaldehyde, to construct the eight-membered ring of the target, unexpectedly provided a cyclopentene (67%), which arises from participation of the C12-α-olefin in the transformation. The diketone derived from the enantiomeric C12-fragment (R)-30 underwent reductive cyclization to provide the desired product in 60% yield. This was elaborated to 12-epi-mutilin by a four-step sequence (39% overall). Installation of the glycolic acid residue followed by C12 epimerization (Berner et al. Monatsh. Chem. 1986, 117, 1073) generated (+)-pleuromutilin (1). (+)-12-epi-Pleuromutilin and (+)-11,12-di-epi-pleuromutilin were prepared by related sequences. This work establishes a convergent entry to the pleuromutilins and provides a foundation for the production of novel antibiotics to treat drug-resistant and Gram-negative infections.
Figures


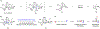


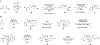








Similar articles
-
A modular and enantioselective synthesis of the pleuromutilin antibiotics.Science. 2017 Jun 2;356(6341):956-959. doi: 10.1126/science.aan0003. Science. 2017. PMID: 28572392 Free PMC article.
-
Metric-Based Analysis of Convergence in Complex Molecule Synthesis.Acc Chem Res. 2021 Feb 16;54(4):903-916. doi: 10.1021/acs.accounts.0c00817. Epub 2021 Feb 1. Acc Chem Res. 2021. PMID: 33523640
-
Total Synthesis of (+)-Pleuromutilin.J Am Chem Soc. 2018 Jan 31;140(4):1267-1270. doi: 10.1021/jacs.7b13260. Epub 2018 Jan 17. J Am Chem Soc. 2018. PMID: 29323492 Free PMC article.
-
Pleuromutilins: Potent Drugs for Resistant Bugs-Mode of Action and Resistance.Cold Spring Harb Perspect Med. 2017 Jan 3;7(1):a027110. doi: 10.1101/cshperspect.a027110. Cold Spring Harb Perspect Med. 2017. PMID: 27742734 Free PMC article. Review.
-
Mutilins derivatives: from veterinary to human-used antibiotics.Mini Rev Med Chem. 2009 Oct;9(12):1397-406. doi: 10.2174/138955709789957387. Mini Rev Med Chem. 2009. PMID: 19929813 Review.
Cited by
-
Scalable Access to Arylomycins via C-H Functionalization Logic.J Am Chem Soc. 2018 Feb 14;140(6):2072-2075. doi: 10.1021/jacs.8b00087. Epub 2018 Feb 6. J Am Chem Soc. 2018. PMID: 29381350 Free PMC article.
-
Total synthesis of structurally diverse pleuromutilin antibiotics.Nat Chem. 2022 Nov;14(11):1270-1277. doi: 10.1038/s41557-022-01027-7. Epub 2022 Sep 26. Nat Chem. 2022. PMID: 36163267 Free PMC article.
-
Strategies to access the [5-8] bicyclic core encountered in the sesquiterpene, diterpene and sesterterpene series.Beilstein J Org Chem. 2023 Mar 3;19:245-281. doi: 10.3762/bjoc.19.23. eCollection 2023. Beilstein J Org Chem. 2023. PMID: 36895430 Free PMC article. Review.
-
Synthesis of Complex Diterpenes: Strategies Guided by Oxidation Pattern Analysis.Acc Chem Res. 2021 Mar 16;54(6):1360-1373. doi: 10.1021/acs.accounts.0c00858. Epub 2021 Feb 23. Acc Chem Res. 2021. PMID: 33621061 Free PMC article.
-
The Latest Progress in the Chemistry of Daphniphyllum Alkaloids.Molecules. 2024 Nov 21;29(23):5498. doi: 10.3390/molecules29235498. Molecules. 2024. PMID: 39683658 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

