The Structure of Human Neuromuscular Junctions: Some Unanswered Molecular Questions
- PMID: 29048368
- PMCID: PMC5666864
- DOI: 10.3390/ijms18102183
The Structure of Human Neuromuscular Junctions: Some Unanswered Molecular Questions
Abstract
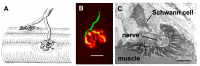
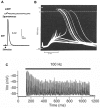
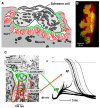
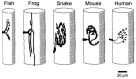
The commands that control animal movement are transmitted from motor neurons to their target muscle cells at the neuromuscular junctions (NMJs). The NMJs contain many protein species whose role in transmission depends not only on their inherent properties, but also on how they are distributed within the complex structure of the motor nerve terminal and the postsynaptic muscle membrane. These molecules mediate evoked chemical transmitter release from the nerve and the action of that transmitter on the muscle. Human NMJs are among the smallest known and release the smallest number of transmitter "quanta". By contrast, they have the most deeply infolded postsynaptic membranes, which help to amplify transmitter action. The same structural features that distinguish human NMJs make them particularly susceptible to pathological processes. While much has been learned about the molecules which mediate transmitter release and action, little is known about the molecular processes that control the growth of the cellular and subcellular components of the NMJ so as to give rise to its mature form. A major challenge for molecular biologists is to understand the molecular basis for the development and maintenance of functionally important aspects of NMJ structure, and thereby to point to new directions for treatment of diseases in which neuromuscular transmission is impaired.
Keywords: disease; human; mouse; neuromuscular junction; neuromuscular transmission; structure.
Conflict of interest statement
The author declares no conflicts of interest.
Figures




References
-
- Salpeter M.M. The Vertebrate Neuromuscular Junction. Alan R. Liss, Inc.; New York, NY, USA: 1986.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

