Obesity-induced vascular dysfunction and arterial stiffening requires endothelial cell arginase 1
- PMID: 29048462
- PMCID: PMC6410953
- DOI: 10.1093/cvr/cvx164
Obesity-induced vascular dysfunction and arterial stiffening requires endothelial cell arginase 1
Erratum in
-
Obesity-induced vascular dysfunction and arterial stiffening requires endothelial cell arginase 1.Cardiovasc Res. 2018 Jan 1;114(1):64. doi: 10.1093/cvr/cvx217. Cardiovasc Res. 2018. PMID: 29182768 Free PMC article. No abstract available.
Abstract
Aims: Elevation of arginase activity has been linked to vascular dysfunction in diabetes and hypertension by a mechanism involving decreased nitric oxide (NO) bioavailability due to L-arginine depletion. Excessive arginase activity also can drive L-arginine metabolism towards the production of ornithine, polyamines, and proline, promoting proliferation of vascular smooth muscle cells and collagen formation, leading to perivascular fibrosis. We hypothesized that there is a specific involvement of arginase 1 expression within the vascular endothelial cells in this pathology.
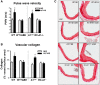
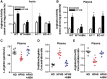
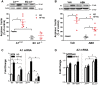
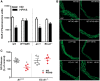
Methods and results: To test this proposition, we used models of type 2 diabetes and metabolic syndrome. Studies were performed using wild type (WT), endothelial-specific arginase 1 knockout (EC-A1-/-) and littermate controls(A1con) mice fed high fat-high sucrose (HFHS) or normal diet (ND) for 6 months and isolated vessels exposed to palmitate-high glucose (PA/HG) media. Some WT mice or isolated vessels were treated with an arginase inhibitor, ABH [2-(S)-amino-6-boronohexanoic acid. In WT mice, the HFHS diet promoted increases in body weight, fasting blood glucose, and post-prandial insulin levels along with arterial stiffening and fibrosis, elevated blood pressure, decreased plasma levels of L-arginine, and elevated L-ornithine. The HFHS diet or PA/HG treatment also induced increases in vascular arginase activity along with oxidative stress, reduced vascular NO levels, and impaired endothelial-dependent vasorelaxation. All of these effects except obesity and hypercholesterolemia were prevented or significantly reduced by endothelial-specific deletion of arginase 1 or ABH treatment.
Conclusion: Vascular dysfunctions in diet-induced obesity are prevented by deletion of arginase 1 in vascular endothelial cells or arginase inhibition. These findings indicate that upregulation of arginase 1 expression/activity in vascular endothelial cells has an integral role in diet-induced cardiovascular dysfunction and metabolic syndrome.
Keywords: Arginase; Diabetes; Fibrosis; Obesity; Vascular dysfunction.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2017. For permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Galassi A, Reynolds K, He J.. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med 2006;119:812–819. - PubMed
-
- Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffe-Scrive M.. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism 1995;44:645–651. - PubMed
-
- Kim TN, Kim S, Yang SJ, Yoo HJ, Seo JA, Kim SG, Kim NH, Baik SH, Choi DS, Choi KM.. Vascular inflammation in patients with impaired glucose tolerance and type 2 diabetes: analysis with 18F-fluorodeoxyglucose positron emission tomography. Circ Cardiovasc Imaging 2010;3:142–148. - PubMed
-
- Jung C, Gonon AT, Sjoquist PO, Lundberg JO, Pernow J.. Arginase inhibition mediates cardioprotection during ischaemia-reperfusion. Cardiovasc Res 2010;85:147–154. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

