The role of toxins in Clostridium difficile infection
- PMID: 29048477
- PMCID: PMC5812492
- DOI: 10.1093/femsre/fux048
The role of toxins in Clostridium difficile infection
Abstract
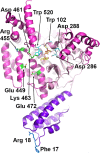
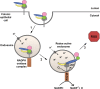
Clostridium difficile is a bacterial pathogen that is the leading cause of nosocomial antibiotic-associated diarrhea and pseudomembranous colitis worldwide. The incidence, severity, mortality and healthcare costs associated with C. difficile infection (CDI) are rising, making C. difficile a major threat to public health. Traditional treatments for CDI involve use of antibiotics such as metronidazole and vancomycin, but disease recurrence occurs in about 30% of patients, highlighting the need for new therapies. The pathogenesis of C. difficile is primarily mediated by the actions of two large clostridial glucosylating toxins, toxin A (TcdA) and toxin B (TcdB). Some strains produce a third toxin, the binary toxin C. difficile transferase, which can also contribute to C. difficile virulence and disease. These toxins act on the colonic epithelium and immune cells and induce a complex cascade of cellular events that result in fluid secretion, inflammation and tissue damage, which are the hallmark features of the disease. In this review, we summarize our current understanding of the structure and mechanism of action of the C. difficile toxins and their role in disease.
Keywords: Clostridium difficile; actin cytoskeleton; bacterial toxins; colitis; glucosyltransferase; inflammation; intestinal epithelium; pore formation.
Published by Oxford University Press on behalf of FEMS 2017.
Figures







References
-
- Aktories K, Barbieri JT. Bacterial cytotoxins: targeting eukaryotic switches. Nat Rev Microbiol 2005;3:397–410. - PubMed
-
- Aktories K, Barmann M, Ohishi I et al. Botulinum C2 toxin ADP-ribosylates actin. Nature 1986;322:390–2. - PubMed
-
- Alcantara C, Stenson WF, Steiner TS et al. Role of inducible cyclooxygenase and prostaglandins in Clostridium difficile toxin A-induced secretion and inflammation in an animal model. J Infect Dis 2001;184:648–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

