Modeling neuro-immune interactions during Zika virus infection
- PMID: 29048558
- PMCID: PMC5886060
- DOI: 10.1093/hmg/ddx382
Modeling neuro-immune interactions during Zika virus infection
Abstract
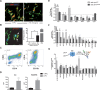
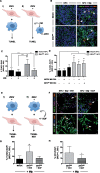
Although Zika virus (ZIKV) infection is often asymptomatic, in some cases, it can lead to birth defects in newborns or serious neurologic complications in adults. However, little is known about the interplay between immune and neural cells that could contribute to the ZIKV pathology. To understand the mechanisms at play during infection and the antiviral immune response, we focused on neural precursor cells (NPCs)-microglia interactions. Our data indicate that human microglia infected with the current circulating Brazilian ZIKV induces a similar pro-inflammatory response found in ZIKV-infected human tissues. Importantly, using our model, we show that microglia interact with ZIKV-infected NPCs and further spread the virus. Finally, we show that Sofosbuvir, an FDA-approved drug for Hepatitis C, blocked viral infection in NPCs and therefore the transmission of the virus from microglia to NPCs. Thus, our model provides a new tool for studying neuro-immune interactions and a platform to test new therapeutic drugs.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Dick G.W.A., kitchen S.F., haddow A.J. (1952) Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg., 46, 509–520. - PubMed
-
- Mlakar J., Korva M., Tul N., Popović M., Poljšak-Prijatelj M., Mraz J., Kolenc M., Resman Rus K., Vesnaver Vipotnik T., Fabjan Vodušek V.. et al. (2016) Zika virus associated with microcephaly. N. Engl. J. Med., 374, 951–958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

