Human cytomegalovirus infection enhances cell proliferation, migration and upregulation of EMT markers in colorectal cancer-derived stem cell-like cells
- PMID: 29048611
- PMCID: PMC5642395
- DOI: 10.3892/ijo.2017.4135
Human cytomegalovirus infection enhances cell proliferation, migration and upregulation of EMT markers in colorectal cancer-derived stem cell-like cells
Abstract
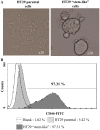
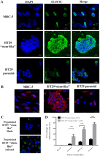
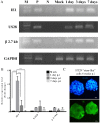
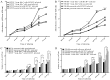
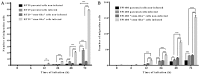
Increasing evidence suggests a link between persistent human cytomegalovirus (HCMV) infection and cancer. Although the role of HCMV in cancer is still elusive, recent studies revealed the presence of HCMV nucleic acids and proteins in different cancer types such as glioblastoma, colorectal, breast, and prostate cancers, and neuroblastoma. Although HCMV may not be directly associated with the neoplastic transformation, the presence of HCMV DNA in the tumorous tissue has been associated with altered clinical outcomes in cancer patients. However, the mechanisms involved in the association between colorectal cancer (CRC) and HCMV are unclear. In this study, we investigated the influence of HCMV infection on CRC or their derived cells. Proliferation and migration assays revealed a high infection efficiency in CRC-derived HT29 and SW480 'stem‑like' cells. After 24, 48 and 72 h of HCMV infection, both HT29 and SW480 parental and stem‑like cells showed a significant increase in cell proliferation and viability (p<0.0001). Moreover, HCMV infection promoted cell migration. These results demonstrate a significant phenotypic alteration in the CRC cell line upon HCMV infection. Using epithelial to mesenchymal transition (EMT) assays, we demonstrated that the EMT markers and driver genes were upregulated during the virus infection. The WNT signaling pathway, which is associated with the proliferation and migration of CRC cells, was upregulated (6-fold) in HCMV-infected cells as compared to the non‑infected cells at day 7 from infection.
Figures
Similar articles
-
Human cytomegalovirus preferentially infects the neoplastic epithelium of colorectal cancer: a quantitative and histological analysis.J Clin Virol. 2012 Jul;54(3):240-4. doi: 10.1016/j.jcv.2012.04.007. Epub 2012 May 15. J Clin Virol. 2012. PMID: 22595308
-
Human cytomegalovirus gene expression in long-term infected glioma stem cells.PLoS One. 2014 Dec 30;9(12):e116178. doi: 10.1371/journal.pone.0116178. eCollection 2014. PLoS One. 2014. PMID: 25549333 Free PMC article.
-
Cadherin-12 enhances proliferation in colorectal cancer cells and increases progression by promoting EMT.Tumour Biol. 2016 Jul;37(7):9077-88. doi: 10.1007/s13277-015-4555-z. Epub 2016 Jan 14. Tumour Biol. 2016. PMID: 26762412 Free PMC article.
-
Polyploid Giant Cancer Cells: A Distinctive Feature in the Transformation of Epithelial Cells by High-Risk Oncogenic HCMV Strains.Viruses. 2024 Jul 31;16(8):1225. doi: 10.3390/v16081225. Viruses. 2024. PMID: 39205199 Free PMC article. Review.
-
Mechanisms of human cytomegalovirus persistence and latency.Front Biosci. 2002 Jun 1;7:d1575-82. doi: 10.2741/jarvis. Front Biosci. 2002. PMID: 12045013 Review.
Cited by
-
Berberine in Human Oncogenic Herpesvirus Infections and Their Linked Cancers.Viruses. 2021 May 28;13(6):1014. doi: 10.3390/v13061014. Viruses. 2021. PMID: 34071559 Free PMC article. Review.
-
The Human Cytomegalovirus, from Oncomodulation to Oncogenesis.Viruses. 2018 Aug 3;10(8):408. doi: 10.3390/v10080408. Viruses. 2018. PMID: 30081496 Free PMC article. Review.
-
Overexpression of the human cytomegalovirus UL111A is correlated with favorable survival of patients with gastric cancer and changes T-cell infiltration and suppresses carcinogenesis.J Cancer Res Clin Oncol. 2020 Mar;146(3):555-568. doi: 10.1007/s00432-019-03092-x. Epub 2020 Feb 5. J Cancer Res Clin Oncol. 2020. PMID: 32025866 Free PMC article.
-
The Human Virome: Viral Metagenomics, Relations with Human Diseases, and Therapeutic Applications.Viruses. 2022 Jan 28;14(2):278. doi: 10.3390/v14020278. Viruses. 2022. PMID: 35215871 Free PMC article. Review.
-
Tumor resident, TRA anti-viral CDR3 chemical sequence motifs are associated with a better breast cancer outcome.Genes Immun. 2023 Apr;24(2):92-98. doi: 10.1038/s41435-023-00201-2. Epub 2023 Feb 18. Genes Immun. 2023. PMID: 36805542
References
-
- World Health Organization fact sheet Cancer. 2017 Feb; http://www.who.int/mediacentre/factsheets/fs297/en/
-
- Moore HG, Baxter NN, Guillem JG. Colorectal cancer: Epidemiology, etiology, and molecular basis. In: Beck D, editor. The ASCRS Textbook of Colon and Rectal Surgery. 2nd edition. Springer Science, Business Media; 2011. pp. 669–690. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

