Dual action of NSC606985 on cell growth and apoptosis mediated through PKCδ in prostatic cancer cells
- PMID: 29048618
- PMCID: PMC5643069
- DOI: 10.3892/ijo.2017.4138
Dual action of NSC606985 on cell growth and apoptosis mediated through PKCδ in prostatic cancer cells
Abstract
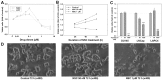
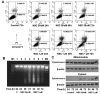
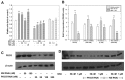
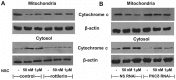
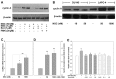
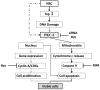
Chemotherapy is a vital therapeutic strategy for castration-resistant prostate cancer (CRPC). We have previously shown that NSC606985 (NSC), a camptothecin (CPT) analog, induced cell apoptosis via interacting with topoisomerase I (Topo I) in prostate cancer cells. In the present study, the effect and mechanism of CPT analogs in LAPC4 cells were investigated. LAPC-4 cells were treated with NSC, CPT, and topotecan. Cell proliferation, apoptosis, and protein kinase Cδ (PKCδ) subcellular activation were measured at different doses and time-points, with or without PKCδ inhibition or knockdown of PKCδ expression. NSC at doses ranging from 10 to 100 nM induced a dose-dependent increase in viable cell number and DNA biosynthesis with mild cell apoptosis, whereas, at doses ranging from 500 nM to 5 mM, NSC produced a dose-dependent decrease in cell proliferation and DNA biosynthesis with a significant induction of cell apoptosis. Both NSC-induced cell proliferation and apoptosis were blocked by knockdown of PKCδ with a specific RNAi, or by the co-administration of rottlerin, a PKCδ inhibitor. Moreover, NSC produced a dose-dependent subcellular activation of PKCδ. The dose-dependent dual action of NSC is mediated at least in part through the differential subcellular activation of PKCδ in LAPC4 cells. The demonstration of a differential cell response to camptothecin analogs would facilitate the identification of biomarker(s) to CPT sensitivity and promote the personalization of CPT chemotherapy in CRPC.
Figures

References
-
- Frese S, Schüller A, Frese-Schaper M, Gugger M, Schmid RA. Cytotoxic effects of camptothecin and cisplatin combined with tumor necrosis factor-related apoptosis-inducing ligand (Apo2L/TRAIL) in a model of primary culture of non-small cell lung cancer. Anticancer Res. 2009;29:2905–2911. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

