Representation of Women and Minorities in Clinical Trials for New Molecular Entities and Original Therapeutic Biologics Approved by FDA CDER from 2013 to 2015
- PMID: 29048983
- PMCID: PMC7001461
- DOI: 10.1089/jwh.2016.6272
Representation of Women and Minorities in Clinical Trials for New Molecular Entities and Original Therapeutic Biologics Approved by FDA CDER from 2013 to 2015
Abstract
Background: The U.S. Food and Drug Administration (FDA) has made efforts to encourage adequate assessment of women, racial/ethnic minorities, and geriatric participants in clinical trials through regulations and guidance documents. This study surveyed the demographics of clinical trial participants and the presence of efficacy and safety analyses by sex for new drugs approved between 2013 and 2015 by the FDA Center for Drug Evaluation and Research.
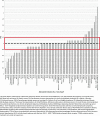
Methods: New drug marketing applications submitted to FDA were surveyed for demographic data (sex, race, ethnicity, and age) and the presence of sex-based analyses for efficacy and safety. The Ratio of the Proportion of women in clinical trials for the indicated disease population relative to the estimated Proportion of women in the disease population (PPR) was calculated for new drug indications.
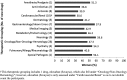
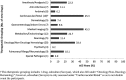
Results: Of the 102 new drugs in this cohort (defined as new molecular entity drugs and original therapeutic biologics), sex was reported for >99.9% of trial participants, and women accounted for 40.4% of these participants. An estimated 77.2% of participants were White, 6.4% were Black/African American, and 29.1% were aged ≥65 years. Sex-based analyses for both efficacy and safety were conducted for 93.1% of applications. PPR was calculated for 82 new drugs for a total of 60 indications, of which 50 indications (83.3%) had a PPR ≥0.80.
Conclusions: Sex data are now collected for almost all study participants, and this study shows appropriate sex participation for most new drugs when estimated disease prevalence by sex (PPR) is considered. Therapeutic area and disease indication are important considerations when assessing the sex of participants because variation occurs depending on the disease under study. Some racial minorities, especially Blacks/African Americans, are still not well represented in most drug development programs and remain an area where improvement is needed.
Keywords: United States Food and Drug Administration (FDA); clinical trial; demography; sex distribution.
Conflict of interest statement
No competing financial interests exist.
Figures


References
-
- Parekh A, Fadiran EO, Uhl J, Throckmorton DC. Adverse effects in women: Implications for drug development and regulatory policies. Expert Rev Clin Pharmacol 2011;4:453–466 - PubMed
-
- Fadiran EO, Zhang L. Effects of sex differences in the pharmacokinetics of drugs and their impact on the safety of medicines in women. In: Harrison-Woolrych M, ed. Medicines for women. Switzerland: Springer International Publishing, 2015:41–68
-
- Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol 2004;44:499–523 - PubMed
-
- Benton RE, Sale M, Flockhart DA, Woosley RL. Greater quinidine-induced QTc interval prolongation in women. Clin Pharmacol Ther 2000;67:413–418 - PubMed
-
- Drici MD, Clément N. Is gender a risk factor for adverse drug reactions? The example of drug-induced long QT syndrome. Drug Saf 2001;24:575–585 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

