Paclitaxel as third-line chemotherapy for small cell lung cancer failing both etoposide- and camptothecin-based chemotherapy
- PMID: 29049199
- PMCID: PMC5662365
- DOI: 10.1097/MD.0000000000008176
Paclitaxel as third-line chemotherapy for small cell lung cancer failing both etoposide- and camptothecin-based chemotherapy
Abstract
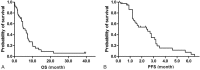
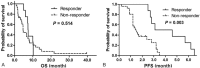
Paclitaxel has been shown to have clinical activity in the treatment of small cell lung cancer (SCLC). However, its role as third-line chemotherapy for SCLC after both etoposide- and camptothecin-based regimens has not been clarified.All patients with refractory SCLC who were treated with paclitaxel-based regimen as third-line chemotherapy between 2005 and 2011 in Seoul National University Bundang Hospital were reviewed retrospectively. Forty patients previously treated with both etoposide- and camptothecin-based chemotherapy were included.The median age of the enrolled patients was 67 years (range, 35-86 years). Most patients (77.5%) received cisplatin plus etoposide as first-line therapy, and camptothecins such as irinotecan or topotecan as second-line therapy. Of 34 patients with measurable lesions, 8 patients (23.5%) achieved partial response and 9 (26.5%) had stable disease. The median progression-free survival (PFS) and overall survival (OS) were 2.5 and 5.9 months, respectively. Predictive factors for OS were performance status (PS) (PS <2 vs ≥2; P = .001), the presence of liver metastasis (P < .001), and number of metastatic sites (<3 vs ≥3; P = .047) in univariate analysis. PS and liver metastasis also remained statistically significant in multivariate analysis. Grade 3 or 4 hematologic toxicity was 20% for neutropenia, and 10% for thrombocytopenia. Other common non-hematological toxicities were peripheral neuropathy and mild liver enzyme elevation.Paclitaxel-based chemotherapy showed modest activity in SCLC patients refractory to both etoposide- and camptothecin-based chemotherapy. PS and presence of liver metastasis were predictive of survival after paclitaxel chemotherapy.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
References
-
- Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011;61:212–36. - PubMed
-
- O’Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006;24:5441–7. - PubMed
-
- Cheng S, Evans WK, Stys-Norman D, et al. Chemotherapy for relapsed small cell lung cancer: a systematic review and practice guideline. J Thorac Oncol 2007;2:348–54. - PubMed
-
- Masters GA, Declerck L, Blanke C, et al. Phase II trial of gemcitabine in refractory or relapsed small-cell lung cancer: Eastern Cooperative Oncology Group Trial 1597. J Clin Oncol 2003;21:1550–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

