Update: Interim Guidance for the Diagnosis, Evaluation, and Management of Infants with Possible Congenital Zika Virus Infection - United States, October 2017
- PMID: 29049277
- PMCID: PMC5689094
- DOI: 10.15585/mmwr.mm6641a1
Update: Interim Guidance for the Diagnosis, Evaluation, and Management of Infants with Possible Congenital Zika Virus Infection - United States, October 2017
Abstract
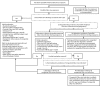
CDC has updated its interim guidance for U.S. health care providers caring for infants with possible congenital Zika virus infection (1) in response to recently published updated guidance for health care providers caring for pregnant women with possible Zika virus exposure (2), unknown sensitivity and specificity of currently available diagnostic tests for congenital Zika virus infection, and recognition of additional clinical findings associated with congenital Zika virus infection. All infants born to mothers with possible Zika virus exposure* during pregnancy should receive a standard evaluation at birth and at each subsequent well-child visit including a comprehensive physical examination, age-appropriate vision screening and developmental monitoring and screening using validated tools (3-5), and newborn hearing screen at birth, preferably using auditory brainstem response (ABR) methodology (6). Specific guidance for laboratory testing and clinical evaluation are provided for three clinical scenarios in the setting of possible maternal Zika virus exposure: 1) infants with clinical findings consistent with congenital Zika syndrome regardless of maternal testing results, 2) infants without clinical findings consistent with congenital Zika syndrome who were born to mothers with laboratory evidence of possible Zika virus infection,† and 3) infants without clinical findings consistent with congenital Zika syndrome who were born to mothers without laboratory evidence of possible Zika virus infection. Infants in the first two scenarios should receive further testing and evaluation for Zika virus, whereas for the third group, further testing and clinical evaluation for Zika virus are not recommended. Health care providers should remain alert for abnormal findings (e.g., postnatal-onset microcephaly and eye abnormalities without microcephaly) in infants with possible congenital Zika virus exposure without apparent abnormalities at birth.
Conflict of interest statement
Figures
Comment in
-
The CDC Updates Guidelines for Congenital Zika.Am J Nurs. 2018 Feb;118(2):14. doi: 10.1097/01.NAJ.0000530232.22909.7c. Am J Nurs. 2018. PMID: 29369860
References
-
- American Academy of Pediatrics, Committee on Practice and Ambulatory Medicine, Section on Ophthalmology, American Association of Certified Orthoptists, American Association for Pediatric Ophthalmology and Strabismus, American Academy of Ophthalmology. Visual system assessment in infants, children, and young adults by pediatricians. Pediatrics 2016;137:e20153596. 10.1542/peds.2015-3596 - DOI - PubMed
-
- Council on Children With Disabilities; Section on Developmental Behavioral Pediatrics; Bright Futures Steering Committee; Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics 2006;118:405–20. 10.1542/peds.2006-1231 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

