XRCC5 cooperates with p300 to promote cyclooxygenase-2 expression and tumor growth in colon cancers
- PMID: 29049411
- PMCID: PMC5648251
- DOI: 10.1371/journal.pone.0186900
XRCC5 cooperates with p300 to promote cyclooxygenase-2 expression and tumor growth in colon cancers
Abstract
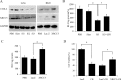
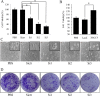
Cyclooxygenase (COX) is the rate-limiting enzyme in prostaglandins (PGs) biosynthesis. Previous studies indicate that COX-2, one of the isoforms of COX, is highly expressed in colon cancers and plays a key role in colon cancer carcinogenesis. Thus, searching for novel transcription factors regulating COX-2 expression will facilitate drug development for colon cancer. In this study, we identified XRCC5 as a binding protein of the COX-2 gene promoter in colon cancer cells with streptavidin-agarose pulldown assay and mass spectrometry analysis, and found that XRCC5 promoted colon cancer growth through modulation of COX-2 signaling. Knockdown of XRCC5 by siRNAs inhibited the growth of colon cancer cells in vitro and of tumor xenografts in a mouse model in vivo by suppressing COX-2 promoter activity and COX-2 protein expression. Conversely, overexpression of XRCC5 promoted the growth of colon cancer cells by activating COX-2 promoter and increasing COX-2 protein expression. Moreover, the role of p300 (a transcription co-activator) in acetylating XRCC5 to co-regulate COX-2 expression was also evaluated. Immunofluorescence assay and confocal microscopy showed that XRCC5 and p300 proteins were co-located in the nucleus of colon cancer cells. Co-immunoprecipitation assay also proved the interaction between XRCC5 and p300 in nuclear proteins of colon cancer cells. Cell viability assay indicated that the overexpression of wild-type p300, but not its histone acetyltransferase (HAT) domain deletion mutant, increased XRCC5 acetylation, thereby up-regulated COX-2 expression and promoted the growth of colon cancer cells. In contrast, suppression of p300 by a p300 HAT-specific inhibitor (C646) inhibited colon cancer cell growth by suppressing COX-2 expression. Taken together, our results demonstrated that XRCC5 promoted colon cancer growth by cooperating with p300 to regulate COX-2 expression, and suggested that the XRCC5/p300/COX-2 signaling pathway was a potential target in the treatment of colon cancers.
Conflict of interest statement
Figures



References
-
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014; 383(9927):1490–1502. doi: 10.1016/S0140-6736(13)61649-9 - DOI - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017; 67(1):7–30. doi: 10.3322/caac.21387 - DOI - PubMed
-
- Prados J, Melguizo C, Ortiz R, Perazzoli G, Cabeza L, Alvarez PJ, et al. Colon cancer therapy: recent developments in nanomedicine to improve the efficacy of conventional chemotherapeutic drugs. Anticancer Agents Med Chem. 2013; 13(8):1204–1216. - PubMed
-
- Ciombor KK, Wu C, Goldberg RM. Recent Therapeutic Advances in the Treatment of Colorectal Cancer. Annual Review of Medicine. 2015; 66:83–95. doi: 10.1146/annurev-med-051513-102539 - DOI - PubMed
-
- Rouzer CA, Marnett LJ. Structural and functional differences between cyclooxygenases: fatty acid oxygenases with a critical role in cell signaling. Biochem Biophys Res Commun. 2005;338(1):34–44. doi: 10.1016/j.bbrc.2005.07.198 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

