Cost-effectiveness of Testing and Treatment for Latent Tuberculosis Infection in Residents Born Outside the United States With and Without Medical Comorbidities in a Simulation Model
- PMID: 29049814
- PMCID: PMC5808933
- DOI: 10.1001/jamainternmed.2017.3941
Cost-effectiveness of Testing and Treatment for Latent Tuberculosis Infection in Residents Born Outside the United States With and Without Medical Comorbidities in a Simulation Model
Abstract
Importance: Testing for and treating latent tuberculosis infection (LTBI) is among the main strategies to achieve TB elimination in the United States. The best approach to testing among non-US born residents, particularly those with comorbid conditions, is uncertain.
Objective: To estimate health outcomes, costs, and cost-effectiveness of LTBI testing and treatment among non-US born residents with and without medical comorbidities.
Design, setting, and participants: Decision analytic tree and Markov cohort simulation model among non-US born residents with no comorbidities, with diabetes, with HIV infection, or with end-stage renal disease (ESRD) using a health care sector perspective with 3% annual discounting. Strategies compared included no testing, tuberculin skin test (TST), interferon gamma release assay (IGRA), confirm positive (initial TST, IGRA only for TST-positive results; both tests positive indicates LTBI), and confirm negative (initial IGRA, then TST for IGRA-negative; any test positive indicates LTBI). All strategies were coupled to treatment with 3 months of self-administered rifapentine and isoniazid.
Main outcomes and measures: Number needed to test and treat to prevent 1 case of TB reactivation, discounted quality-adjusted life-years (QALYs), discounted lifetime medical costs, and incremental cost-effectiveness ratios (ICERs).
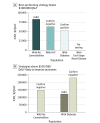
Results: Improving health outcomes increased costs, with choice of test dependent on willingness to pay. Strategies ranked by ascending costs and benefits: no testing, confirm positive, TST, IGRA, and confirm negative. The ICERs varied by non-US born patient risk group: patients with no comorbidities, IGRA was likely cost-effective at $83 000/QALY; patients with diabetes, both confirm positive ($53 000/QALY) and IGRA ($120 000/QALY) were likely cost-effective; patients with HIV, confirm negative was clearly preferred ($63 000/QALY); and patients with ESRD, no testing was cost-effective. Increased LTBI prevalence and reduced return for TST reading improved IGRA's relative performance. In 10 000 probabilistic simulations among non-US born patients with no comorbidities, with diabetes, and with HIV, some form of testing was virtually always cost-effective. These simulations highlight the uncertainty of test choice for non-US born patients with no comorbidities and non-US born patients with diabetes, but strategies including IGRA were preferred in over 60% of simulations for all non-US born populations except those with ESRD.
Conclusions and relevance: Testing for and treating LTBI among non-US born residents with and without selected comorbidities is likely cost-effective except among those with ESRD in whom competing risks of death limit benefits. Strategies including IGRA fell below a $100 000/QALY willingness-to-pay threshold for non-US born patients with no comorbidities, patients with diabetes, and patients with HIV.
Conflict of interest statement
Figures


Comment in
-
Mainstreaming Latent Tuberculosis Infection Testing and Treatment in the United States: Who and How.JAMA Intern Med. 2017 Dec 1;177(12):1764-1765. doi: 10.1001/jamainternmed.2017.4916. JAMA Intern Med. 2017. PMID: 29049821 No abstract available.
References
-
- Centers for Disease Control and Prevention. Reported tuberculosis in the United States, 2014. https://www.cdc.gov/tb/statistics/reports/2014/pdfs/tb-surveillance-2014.... Published October 2015. Accessed January 15, 2017.
-
- Sterling TR, Villarino ME, Borisov AS, et al. ; TB Trials Consortium PREVENT TB Study Team . Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365(23):2155-2166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

