Optimal dosing and delivery of parathyroid hormone and its analogues for osteoporosis and hypoparathyroidism - translating the pharmacology
- PMID: 29049872
- PMCID: PMC5777439
- DOI: 10.1111/bcp.13455
Optimal dosing and delivery of parathyroid hormone and its analogues for osteoporosis and hypoparathyroidism - translating the pharmacology
Abstract
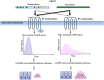
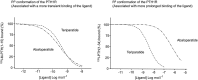
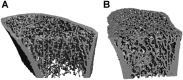
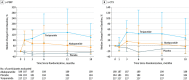
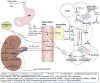
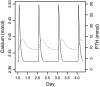
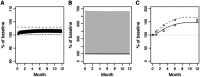
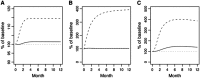
In primary hyperparathyroidism (PHPT), bone loss results from the resorptive effects of excess parathyroid hormone (PTH). Under physiological conditions, PTH has actions that are more targeted to homeostasis and to bone accrual. The predominant action of PTH, either catabolic, anabolic or homeostatic, can be understood in molecular and pharmacokinetic terms. When administered intermittently, PTH increases bone mass, but when present continuously and in excess (e.g. PHPT), bone loss ensues. This dual effect of PTH depends not only on the dosing regimen, continuous or intermittent, but also on how the PTH molecule interacts with various states of its receptor (PTH/PTHrP receptor) influencing downstream signalling pathways differentially. Altering the amino-terminal end of PTH or PTHrP could emphasize the state of the receptor that is linked to an osteoanabolic outcome. This concept led to the development of a PTHrP analogue that interacts preferentially with the transiently linked state of the receptor, emphasizing an osteoanabolic effect. However, designing PTH or PTHrP analogues with prolonged state of binding to the receptor would be expected to be linked to a homeostatic action associated with the tonic secretory state of the parathyroid glands that is advantageous in treating hypoparathyroidism. Ideally, further development of a drug delivery system that mimics the physiological tonic, circadian, and pulsatile profile of PTH would be optimal. This review discusses basic, translational and clinical studies that may well lead to newer approaches to the treatment of osteoporosis as well as to different PTH molecules that could become more advantageous in treating hypoparathyroidism.
Keywords: PTH; PTHrP; abaloparatide; clinical pharmacology; hypoparathyroidism; osteoporosis; parathyroid hormone; primary hyperparathyroidism.
© 2017 The British Pharmacological Society.
Figures
References
-
- Kempson SA, Lotscher M, Kaissling B, Biber J, Murer H, Levi M. Parathyroid hormone action on phosphate transporter mRNA and protein in rat renal proximal tubules. Am J Physiol 1995; 268 (4 Pt 2): F784–F791. - PubMed
-
- Silva BC, Kousteni S. Chapter 8 – cellular actions of PTH: osteoblasts, osteoclasts, and osteocytes A2. In: Bilezikian In: The Parathyroids, Third edn, ed JP San Diego: Academic Press, 2015; 127–137.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

