LncRNAs: key players and novel insights into diabetes mellitus
- PMID: 29050364
- PMCID: PMC5642639
- DOI: 10.18632/oncotarget.19921
LncRNAs: key players and novel insights into diabetes mellitus
Abstract
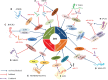
Long non-coding RNAs (LncRNAs) are a class of endogenous RNA molecules, which have a transcribing length of over 200 nt, lack a complete functional open reading frame (ORF), and rarely encode a functional short peptide. Recent studies have revealed that disruption of LncRNAs levels correlates with several human diseases, including diabetes mellitus (DM), a complex multifactorial metabolic disorder affecting more than 400 million people worldwide. LncRNAs are emerging as pivotal regulators in various biological processes, in the progression of DM and its associated complications, involving pancreatic β-cell disorder, insulin resistance, and epigenetic regulation, etc. Further investigation into the mechanisms of action of LncRNAs in DM will be of great value in the thorough understanding of pathogenesis. However, prior to successful application of LncRNAs, further search for molecular biomarkers and drug targets to provide a new strategy for DM prevention, early diagnosis, and therapy is warranted.
Keywords: LncRNAs; diabetes mellitus; epigenetic regulation; insulin resistance; pancreatic β cells.
Conflict of interest statement
CONFLICTS OF INTEREST None.
Figures
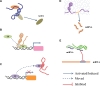
 ”represents activated or induced; the symbol “
”represents activated or induced; the symbol “ ”represents inhibited; and the symbol “
”represents inhibited; and the symbol “ ” represents combined or sponged.
” represents combined or sponged.References
-
- Paulson H, Gonzalez-Alegre P. RNAi gets its prize. Lancet Neurol. 2006;5:997–999. - PubMed
-
- Yin Y, Yan P, Lu J, Song G, Zhu Y, Li Z, Zhao Y, Shen B, Huang X, Zhu H, Orkin SH, Shen X. Opposing Roles for the lncRNA Haunt and Its Genomic Locus in Regulating HOXA Gene Activation during Embryonic Stem Cell Differentiation. Cell Stem Cell. 2015;16:504–516. - PubMed
-
- Gong Z, Zhang S, Zhang W, Huang H, Li Q, Deng H, Ma J, Zhou M, Xiang J, Wu M, Li X, Xiong W, Li X, et al. Long non-coding RNAs in cancer. Sci China Life Sci. 2012;55:1120–1124. - PubMed
-
- Peng L, Yuan X, Jiang B, Tang Z, Li GC. LncRNAs: key players and novel insights into cervical cancer. Tumour Biol. 2016;37:2779–2788. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

