Delineating SPTAN1 associated phenotypes: from isolated epilepsy to encephalopathy with progressive brain atrophy
- PMID: 29050398
- PMCID: PMC6248409
- DOI: 10.1093/brain/awx195
Delineating SPTAN1 associated phenotypes: from isolated epilepsy to encephalopathy with progressive brain atrophy
Abstract
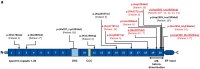
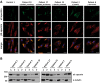
De novo in-frame deletions and duplications in the SPTAN1 gene, encoding the non-erythrocyte αII spectrin, have been associated with severe West syndrome with hypomyelination and pontocerebellar atrophy. We aimed at comprehensively delineating the phenotypic spectrum associated with SPTAN1 mutations. Using different molecular genetic techniques, we identified 20 patients with a pathogenic or likely pathogenic SPTAN1 variant and reviewed their clinical, genetic and imaging data. SPTAN1 de novo alterations included seven unique missense variants and nine in-frame deletions/duplications of which 12 were novel. The recurrent three-amino acid duplication p.(Asp2303_Leu2305dup) occurred in five patients. Our patient cohort exhibited a broad spectrum of neurodevelopmental phenotypes, comprising six patients with mild to moderate intellectual disability, with or without epilepsy and behavioural disorders, and 14 patients with infantile epileptic encephalopathy, of which 13 had severe neurodevelopmental impairment and four died in early childhood. Imaging studies suggested that the severity of neurological impairment and epilepsy correlates with that of structural abnormalities as well as the mutation type and location. Out of seven patients harbouring mutations outside the α/β spectrin heterodimerization domain, four had normal brain imaging and three exhibited moderately progressive brain and/or cerebellar atrophy. Twelve of 13 patients with mutations located within the spectrin heterodimer contact site exhibited severe and progressive brain, brainstem and cerebellar atrophy, with hypomyelination in most. We used fibroblasts from five patients to study spectrin aggregate formation by Triton-X extraction and immunocytochemistry followed by fluorescence microscopy. αII/βII aggregates and αII spectrin in the insoluble protein fraction were observed in fibroblasts derived from patients with the mutations p.(Glu2207del), p.(Asp2303_Leu2305dup) and p.(Arg2308_Met2309dup), all falling in the nucleation site of the α/β spectrin heterodimer region. Molecular modelling of the seven SPTAN1 amino acid changes provided preliminary evidence for structural alterations of the A-, B- and/or C-helices within each of the mutated spectrin repeats. We conclude that SPTAN1-related disorders comprise a wide spectrum of neurodevelopmental phenotypes ranging from mild to severe and progressive. Spectrin aggregate formation in fibroblasts with mutations in the α/β heterodimerization domain seems to be associated with a severe neurodegenerative course and suggests that the amino acid stretch from Asp2303 to Met2309 in the α20 repeat is important for α/β spectrin heterodimer formation and/or αII spectrin function.
Keywords: West syndrome; epileptic encephalopathy; hypomyelination; myoclonic epilepsy; pontocerebellar atrophy.
© The Author (2017). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

References
-
- An XL, Guo XH, Yang Y, Gratzer WB, Baines AJ, Mohandas N. Inter-subunit interactions in erythroid and non-erythroid spectrins. Biochim Biophys Acta 2011; 1814: 420–7. - PubMed
-
- Bagnall RD, Crompton DE, Petrovski S, Lam L, Cutmore C, Garry SI, et al.Exome-based analysis of cardiac arrhythmia, respiratory control, and epilepsy genes in sudden unexpected death in epilepsy. Ann Neurol 2016; 79: 522–34. - PubMed
-
- Begg GE, Harper SL, Morris MB, Speicher DW. Initiation of spectrin dimerization involves complementary electrostatic interactions between paired triple-helical bundles. J Biol Chem 2000; 275: 3279–87. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

