Neuroimaging findings in infantile Pompe patients treated with enzyme replacement therapy
- PMID: 29050825
- PMCID: PMC5808895
- DOI: 10.1016/j.ymgme.2017.10.005
Neuroimaging findings in infantile Pompe patients treated with enzyme replacement therapy
Abstract
Background: Recombinant human acid α-glucosidase (rhGAA) enzyme replacement therapy (ERT) has prolonged survival in infantile Pompe disease (IPD), but has unmasked central nervous system (CNS) changes.
Methods: Brain imaging, consisting of computed tomography (CT) and/or magnetic resonance imaging (MRI), was performed on 23 patients with IPD (17 CRIM-positive, 6 CRIM-negative) aged 2-38months. Most patients had baseline neuroimaging performed prior to the initiation of ERT. Follow-up neuroimaging was performed in eight.
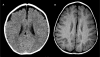
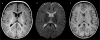
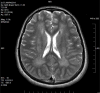
Results: Sixteen patients (70%) had neuroimaging abnormalities consisting of ventricular enlargement (VE) and/or extra-axial cerebrospinal fluid accumulation (EACSF) at baseline, with delayed myelination in two. Follow-up neuroimaging (n=8) after 6-153months showed marked improvement, with normalization of VE and EACSF in seven patients. Two of three patients imaged after age 10years demonstrated white matter changes, with one noted to have a basilar artery aneurysm.
Conclusions: Mild abnormalities on brain imaging in untreated or newly treated patients with IPD tend to resolve with time, in conjunction with ERT. However, white matter changes are emerging as seen in Patients 1 and 3 which included abnormal periventricular white matter changes with subtle signal abnormalities in the basal ganglia and minimal, symmetric signal abnormalities involving the deep frontoparietal cerebral white matter, respectively. The role of neuroimaging as part of the clinical evaluation of IPD needs to be considered to assess for white matter changes and cerebral aneurysms.
Keywords: Acid maltase deficiency; Alglucosidase alfa; Blood-brain barrier; CT; Central nervous system; Enzyme replacement therapy; Glycogen storage disease type II; MRI; Neuroimaging; Neuromuscular diseases; Pompe disease; rhGAA.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
PSK has received research/grant support and honoraria from Genzyme Corporation and Amicus Therapeutics. PSK is a member of the Pompe and Gaucher Disease Registry Advisory Board for Genzyme Corporation. LDH has received research/grant support and honoraria from Genzyme, a Sanofi Company. LDH has also received a consultant fee for Wiley for role as Associate Editor of Muscle & Nerve. PM has received speaker honorarium from Genzyme as a participant in Genzyme’s Patient Speakers Bureau. ZBK, SP, SB, SA, RW, DE and DF do not have conflicts to disclose.
Figures
References
-
- Kishnani PS, et al. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J Pediatr. 2006;148(5):671–676. - PubMed
-
- Marsden D. Infantile onset Pompe disease: a report of physician narratives from an epidemiologic study. Genet Med. 2005;7(2):147–50. - PubMed
-
- van den Hout HM, et al. The natural course of infantile Pompe’s disease: 20 original cases compared with 133 cases from the literature. Pediatrics. 2003;112(2):332–40. - PubMed
-
- Hirschhorn R, Reuser AJJ. Glycogen Storage Disease Type II: Acid a-Glucosidase (Acid Maltase) Deficiency. In: Valle D, Scriver CR, editors. Scriver’s OMMBID the online metabolic & molecular bases of inherited disease. McGraw-Hill; New York: 2010.
-
- Gambetti P, DiMauro S, Baker L. Nervous system in Pompe’s disease. Ultrastructure and biochemistry. J Neuropathol Exp Neurol. 1971;30(3):412–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

