Expansion of EPOR-negative macrophages besides erythroblasts by elevated EPOR signaling in erythrocytosis mouse models
- PMID: 29051279
- PMCID: PMC5777189
- DOI: 10.3324/haematol.2017.172775
Expansion of EPOR-negative macrophages besides erythroblasts by elevated EPOR signaling in erythrocytosis mouse models
Abstract
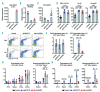
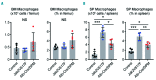
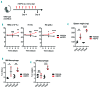
Activated erythropoietin (EPO) receptor (EPOR) signaling causes erythrocytosis. The important role of macrophages for the erythroid expansion and differentiation process has been reported, both in baseline and stress erythropoiesis. However, the significance of EPOR signaling for regulation of macrophages contributing to erythropoiesis has not been fully understood. Here we show that EPOR signaling activation quickly expands both erythrocytes and macrophages in vivo in mouse models of primary and secondary erythrocytosis. To mimic the chimeric condition and expansion of the disease clone in the polycythemia vera patients, we combined Cre-inducible Jak2V617F/+ allele with LysM-Cre allele which expresses in mature myeloid cells and some of the HSC/Ps (LysM-Cre;Jak2V617F/+ mice). We also generated inducible EPO-mediated secondary erythrocytosis models using Alb-Cre, Rosa26-loxP-stop-loxP-rtTA, and doxycycline inducible EPAS1-double point mutant (DPM) alleles (Alb-Cre;DPM mice). Both models developed a similar degree of erythrocytosis. Macrophages were also increased in both models without increase of major inflammatory cytokines and chemokines. EPO administration also quickly induced these macrophages in wild-type mice before observable erythrocytosis. These findings suggest that EPOR signaling activation could induce not only erythroid cell expansion, but also macrophages. Surprisingly, an in vivo genetic approach indicated that most of those macrophages do not express EPOR, but erythroid cells and macrophages contacted tightly with each other. Given the importance of the central macrophages as a niche for erythropoiesis, further elucidation of the EPOR signaling mediated-regulatory mechanisms underlying macrophage induction might reveal a potential therapeutic target for erythrocytosis.
Copyright© 2018 Ferrata Storti Foundation.
Figures



References
-
- Miyake T, Kung CK, Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977;252(15):5558–5564. - PubMed
-
- Jacobs K, Shoemaker C, Rudersdorf R, et al. Isolation and characterization of genomic and cDNA clones of human erythropoietin. Nature. 1985;313(6005):806–810. - PubMed
-
- D’Andrea AD, Lodish HF, Wong GG. Expression cloning of the murine erythropoietin receptor. Cell. 1989;57(2):277–285. - PubMed
-
- Constantinescu SN, Wu H, Liu X, Beyer W, Fallon A, Lodish HF. The anemic Friend virus gp55 envelope protein induces erythroid differentiation in fetal liver colony-forming units-erythroid. Blood. 1998; 91(4):1163–1172. - PubMed
-
- Witthuhn BA, Quelle FW, Silvennoinen O, et al. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74(2):227–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

