Exercise limitations in heart failure with reduced and preserved ejection fraction
- PMID: 29051336
- PMCID: PMC5866447
- DOI: 10.1152/japplphysiol.00747.2017
Exercise limitations in heart failure with reduced and preserved ejection fraction
Abstract
The hallmark symptom of chronic heart failure (HF) is severe exercise intolerance. Impaired perfusive and diffusive O2 transport are two of the major determinants of reduced physical capacity and lowered maximal O2 uptake in patients with HF. It has now become evident that this syndrome manifests at least two different phenotypic variations: heart failure with preserved or reduced ejection fraction (HFpEF and HFrEF, respectively). Unlike HFrEF, however, there is currently limited understanding of HFpEF pathophysiology, leading to a lack of effective pharmacological treatments for this subpopulation. This brief review focuses on the disturbances within the O2 transport pathway resulting in limited exercise capacity in both HFpEF and HFrEF. Evidence from human and animal research reveals HF-induced impairments in both perfusive and diffusive O2 conductances identifying potential targets for clinical intervention. Specifically, utilization of different experimental approaches in humans (e.g., small vs. large muscle mass exercise) and animals (e.g., intravital microscopy and phosphorescence quenching) has provided important clues to elucidating these pathophysiological mechanisms. Adaptations within the skeletal muscle O2 delivery-utilization system following established and emerging therapies (e.g., exercise training and inorganic nitrate supplementation, respectively) are discussed. Resolution of the underlying mechanisms of skeletal muscle dysfunction and exercise intolerance is essential for the development and refinement of the most effective treatments for patients with HF.
Keywords: HFpEF; HFrEF; diffusive O2 transport; exercise intolerance; exercise training; heart failure; perfusive O2 transport; skeletal muscle microcirculation.
Figures
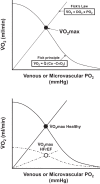


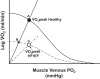





References
-
- Adams V, Alves M, Fischer T, Rolim N, Werner S, Schütt N, Bowen TS, Linke A, Schuler G, Wisloff U. High-intensity interval training attenuates endothelial dysfunction in a Dahl salt-sensitive rat model of heart failure with preserved ejection fraction. J Appl Physiol (1985) 119: 745–752, 2015. doi:10.1152/japplphysiol.01123.2014. - DOI - PubMed
-
- Agnoletti L, Curello S, Bachetti T, Malacarne F, Gaia G, Comini L, Volterrani M, Bonetti P, Parrinello G, Cadei M, Grigolato PG, Ferrari R. Serum from patients with severe heart failure downregulates eNOS and is proapoptotic: role of tumor necrosis factor-α. Circulation 100: 1983–1991, 1999. doi:10.1161/01.CIR.100.19.1983. - DOI - PubMed
-
- Barrett-O’Keefe Z, Lee JF, Berbert A, Witman MA, Nativi-Nicolau J, Stehlik J, Richardson RS, Wray DW. Hemodynamic responses to small muscle mass exercise in heart failure patients with reduced ejection fraction. Am J Physiol Heart Circ Physiol 307: H1512–H1520, 2014. doi:10.1152/ajpheart.00527.2014. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

