Adrenergic nerves activate an angio-metabolic switch in prostate cancer
- PMID: 29051371
- PMCID: PMC5783182
- DOI: 10.1126/science.aah5072
Adrenergic nerves activate an angio-metabolic switch in prostate cancer
Abstract
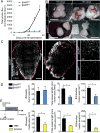
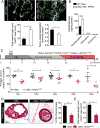
Nerves closely associate with blood vessels and help to pattern the vasculature during development. Recent work suggests that newly formed nerve fibers may regulate the tumor microenvironment, but their exact functions are unclear. Studying mouse models of prostate cancer, we show that endothelial β-adrenergic receptor signaling via adrenergic nerve-derived noradrenaline in the prostate stroma is critical for activation of an angiogenic switch that fuels exponential tumor growth. Mechanistically, this occurs through alteration of endothelial cell metabolism. Endothelial cells typically rely on aerobic glycolysis for angiogenesis. We found that the loss of endothelial Adrb2, the gene encoding the β2-adrenergic receptor, leads to inhibition of angiogenesis through enhancement of endothelial oxidative phosphorylation. Codeletion of Adrb2 and Cox10, a gene encoding a cytochrome IV oxidase assembly factor, prevented the metabolic shift induced by Adrb2 deletion and rescued prostate cancer progression. This cross-talk between nerves and endothelial metabolism could potentially be targeted as an anticancer therapy.
Copyright © 2017 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures


Comment in
-
Nerves switch on angiogenic metabolism.Science. 2017 Oct 20;358(6361):305-306. doi: 10.1126/science.aaq0365. Science. 2017. PMID: 29051365 No abstract available.
-
Tumour angiogenesis: Controlling nerves.Nat Rev Cancer. 2017 Dec;17(12):708. doi: 10.1038/nrc.2017.107. Epub 2017 Nov 10. Nat Rev Cancer. 2017. PMID: 29123247 No abstract available.
-
Prostate cancer: A nervous disposition: the angiometabolic switch.Nat Rev Urol. 2018 Jan;15(1):2. doi: 10.1038/nrurol.2017.200. Epub 2017 Nov 21. Nat Rev Urol. 2018. PMID: 29160866 No abstract available.
-
The Sympathetic Nervous System Drives Tumor Angiogenesis.Trends Cancer. 2018 Feb;4(2):93-94. doi: 10.1016/j.trecan.2017.11.008. Epub 2017 Dec 9. Trends Cancer. 2018. PMID: 29458965
References
-
- Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58–61. - PubMed
-
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nature reviews Cancer. 2003;3:401–410. - PubMed
-
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

