Normal and inverted regimes of charge transfer controlled by density of states at polymer electrodes
- PMID: 29051498
- PMCID: PMC5715087
- DOI: 10.1038/s41467-017-01264-2
Normal and inverted regimes of charge transfer controlled by density of states at polymer electrodes
Abstract
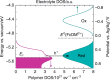
Conductive polymer electrodes have exceptional promise for next-generation bioelectronics and energy conversion devices due to inherent mechanical flexibility, printability, biocompatibility, and low cost. Conductive polymers uniquely exhibit hybrid electronic-ionic transport properties that enable novel electrochemical device architectures, an advantage over inorganic counterparts. Yet critical structure-property relationships to control the potential-dependent rates of charge transfer at polymer/electrolyte interfaces remain poorly understood. Herein, we evaluate the kinetics of charge transfer between electrodeposited poly-(3-hexylthiophene) films and a model redox-active molecule, ferrocenedimethanol. We show that the kinetics directly follow the potential-dependent occupancy of electronic states in the polymer. The rate increases then decreases with potential (both normal and inverted kinetic regimes), a phenomenon distinct from inorganic semiconductors. This insight can be invoked to design polymer electrodes with kinetic selectivity toward redox active species and help guide synthetic approaches for the design of alternative device architectures and approaches.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
-
- Tybrandt K, et al. Translating electronic currents to precise acetylcholine-induced neuronal signaling using an organic electrophoretic delivery device. Adv. Mater. 2009;21:4442–4446. doi: 10.1002/adma.200900187. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

