Recombinant Incretin-Secreting Microbe Improves Metabolic Dysfunction in High-Fat Diet Fed Rodents
- PMID: 29051554
- PMCID: PMC5648875
- DOI: 10.1038/s41598-017-14010-x
Recombinant Incretin-Secreting Microbe Improves Metabolic Dysfunction in High-Fat Diet Fed Rodents
Erratum in
-
Author Correction: Recombinant Incretin-Secreting Microbe Improves Metabolic Dysfunction in High-Fat Diet Fed Rodents.Sci Rep. 2020 Feb 6;10(1):2392. doi: 10.1038/s41598-020-59133-w. Sci Rep. 2020. PMID: 32024931 Free PMC article.
Abstract
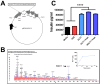
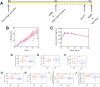
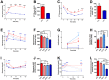
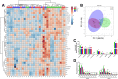
The gut hormone glucagon-like peptide (GLP)-1 and its analogues represent a new generation of anti-diabetic drugs, which have also demonstrated propensity to modulate host lipid metabolism. Despite this, drugs of this nature are currently limited to intramuscular administration routes due to intestinal degradation. The aim of this study was to design a recombinant microbial delivery vector for a GLP-1 analogue and assess the efficacy of the therapeutic in improving host glucose, lipid and cholesterol metabolism in diet induced obese rodents. Diet-induced obese animals received either Lactobacillus paracasei NFBC 338 transformed to express a long-acting analogue of GLP-1 or the isogenic control microbe which solely harbored the pNZ44 plasmid. Short-term GLP-1 microbe intervention in rats reduced serum low-density lipoprotein cholesterol, triglycerides and triglyceride-rich lipoprotein cholesterol substantially. Conversely, extended GLP-1 microbe intervention improved glucose-dependent insulin secretion, glucose metabolism and cholesterol metabolism, compared to the high-fat control group. Interestingly, the microbe significantly attenuated the adiposity associated with the model and altered the serum lipidome, independently of GLP-1 secretion. These data indicate that recombinant incretin-secreting microbes may offer a novel and safe means of managing cholesterol metabolism and diet induced dyslipidaemia, as well as insulin sensitivity in metabolic dysfunction.
Conflict of interest statement
RJS receives compensation for consultancy work as a member of scientific advisory board or research support from Ethicon Endo-Surgery, Novo Nordisk, Novartis, Paul Hastings Law Firm, Zafgen, MedImmune and Sanofi. All other authors declare no duality of interest.
Figures

References
-
- Svegliati-Baroni G, et al. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver international: official journal of the International Association for the Study of the Liver. 2011;31:1285–1297. doi: 10.1111/j.1478-3231.2011.02462.x. - DOI - PubMed
-
- Näslund E, et al. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1999;277:R910–R916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

