Red Blood Cells Homeostatically Bind Mitochondrial DNA through TLR9 to Maintain Quiescence and to Prevent Lung Injury
- PMID: 29053005
- PMCID: PMC5821907
- DOI: 10.1164/rccm.201706-1161OC
Red Blood Cells Homeostatically Bind Mitochondrial DNA through TLR9 to Maintain Quiescence and to Prevent Lung Injury
Abstract
Rationale: Potentially hazardous CpG-containing cell-free mitochondrial DNA (cf-mtDNA) is routinely released into the circulation and is associated with morbidity and mortality in critically ill patients. How the body avoids inappropriate innate immune activation by cf-mtDNA remains unknown. Because red blood cells (RBCs) modulate innate immune responses by scavenging chemokines, we hypothesized that RBCs may attenuate CpG-induced lung inflammation through direct scavenging of CpG-containing DNA.
Objectives: To determine the mechanisms of CpG-DNA binding to RBCs and the effects of RBC-mediated DNA scavenging on lung inflammation.
Methods: mtDNA on murine RBCs was measured under basal conditions and after systemic inflammation. mtDNA content on human RBCs from healthy control subjects and trauma patients was measured. Toll-like receptor 9 (TLR9) expression on RBCs and TLR9-dependent binding of CpG-DNA to RBCs were determined. A murine model of RBC transfusion after CpG-DNA-induced lung injury was used to investigate the role of RBC-mediated DNA scavenging in mitigating lung injury in vivo.
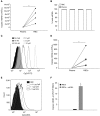
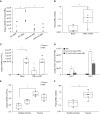
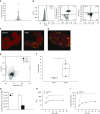
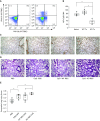
Measurements and main results: Under basal conditions, RBCs bind CpG-DNA. The plasma-to-RBC mtDNA ratio is low in naive mice and in healthy volunteers but increases after systemic inflammation, demonstrating that the majority of cf-mtDNA is RBC-bound under homeostatic conditions and that the unbound fraction increases during inflammation. RBCs express TLR9 and bind CpG-DNA through TLR9. Loss of TLR9-dependent RBC-mediated CpG-DNA scavenging increased lung injury in vivo.
Conclusions: RBCs homeostatically bind mtDNA, and RBC-mediated DNA scavenging is essential in mitigating lung injury after CpG-DNA. Our data suggest a role for RBCs in regulating lung inflammation during disease states where cf-mtDNA is elevated, such as sepsis and trauma.
Keywords: CpG-DNA; RBC; Toll-like receptor 9; mitochondrial DNA.
Figures
Comment in
-
Sequestering Damage-associated Molecular Patterns in Critical Illness. A Novel Homeostatic Role for the Erythrocyte.Am J Respir Crit Care Med. 2018 Feb 15;197(4):416-418. doi: 10.1164/rccm.201710-2094ED. Am J Respir Crit Care Med. 2018. PMID: 29120664 Free PMC article. No abstract available.
References
-
- Stroun M, Lyautey J, Lederrey C, Olson-Sand A, Anker P. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin Chim Acta. 2001;313:139–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

