The evidence on effectiveness of weekly vs triweekly cisplatin concurrent with radiotherapy in locally advanced head and neck squamous cell carcinoma (HNSCC): a systematic review and meta-analysis
- PMID: 29053029
- PMCID: PMC5963366
- DOI: 10.1259/bjr.20170442
The evidence on effectiveness of weekly vs triweekly cisplatin concurrent with radiotherapy in locally advanced head and neck squamous cell carcinoma (HNSCC): a systematic review and meta-analysis
Abstract
Objective: This study aims to synthesize the current available evidences on the effectiveness of weekly vs triweekly cisplatin concurrent with radiotherapy in the primary and adjuvant treatment of locally advanced head and neck squamous cell carcinoma (HNSCC).
Methods: A systematic review and meta-analysis of literature were undertaken to assess the effectiveness of weekly vs triweekly schedule in primary and adjuvant treatment for HNSCC with adverse risk features. Search of relevant articles from electronic database from 2000 to March 2016 and appraisal of studies were done.
Results: Only one randomized controlled trial (RCT) and six retrospective studies were included in this review. The RCT showed less severe mucositis (75 vs 38.5%, p = 0.012) and more patients receiving at least 200 mg/m2 (62.5% vs 88.5%, p = 0.047) of cisplatin in triweekly arm. There was no difference in 1-year progression-free survival (60% vs 71.1%, p = 0.806) and 1-year overall survival (OS) (71.6 vs 79.3%, p = 0.978) between the weekly and triweekly arm. Pooling of data from six studies showed no difference in 5-year progression-free survival (RR 0.84, 95%, CI 0.67-1.07), 5-year OS (RR 0.88, 95% CI 0.73-1.07), severe renal events (RR 0.66, 95% CI 0.42-1.04), severe mucositis (RR 0.92, 95% CI 0.71-1.21), severe dermatitis (RR 0.61, 95% CI 0.37-1.03), treatment interruptions (RR 1.06, 95% CI 0.74-1.52) and number of patients receiving at least 200 mg/m2 (RR 0.83, 95% CI 0.67-1.03).
Conclusion: The current evidence showed that weekly schedule is not superior to triweekly in improving oncological outcomes and decreasing early effects of treatment. In the absence of compelling data, triweekly schedule should remain the standard of care while more RCTs are warranted. Advances in knowledge: While some have proposed that low-dose weekly cisplatin is safer and less toxic, this study emphasized that there is no difference in acute toxicity of the two schedules and it is safe to utilize high-dose cisplatin every 3 weeks to reach the threshold dose of 200 mg/m2 faster. Uniquely, this study excluded nasopharyngeal cancer patients as the biology and treatment response are different with other HNSCC.
Figures
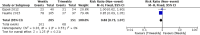
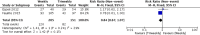


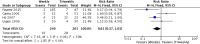
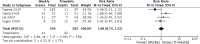

References
-
- NationalComprehensive Cancer Network. Head and Neck Cancer (Version 2.2017). 2017. Available from: https://www.nccn.org/professionals/physician_gls/PDF/head-and-neck.pdf [Cited: 28 March 2017]
-
- Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005; 27: 843–50. DOI: 10.1002/hed.20279 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

