Chiral Catenanes and Rotaxanes: Fundamentals and Emerging Applications
- PMID: 29053181
- PMCID: PMC5923600
- DOI: 10.1002/chem.201704149
Chiral Catenanes and Rotaxanes: Fundamentals and Emerging Applications
Abstract
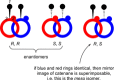
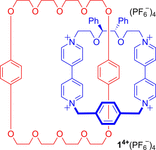
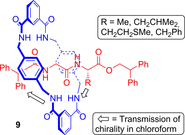
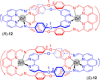
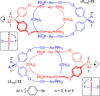
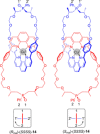
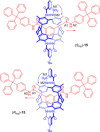
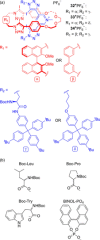
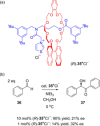
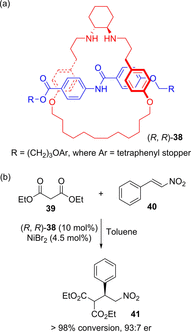
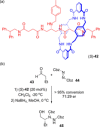
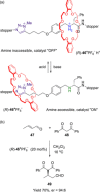
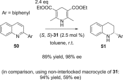
Molecular chirality provides a key challenge in host-guest recognition and other related chemical applications such as asymmetric catalysis. For a molecule to act as an efficient enantioselective receptor, it requires multi-point interactions between host and chiral guest, which may be achieved by an appropriate chiral 3D scaffold. As a consequence of their interlocked structure, catenanes and rotaxanes may present such a 3D scaffold, and can be chiral by inclusion of a classical chiral element and/or as a consequence of the mechanical bond. This Minireview presents illustrative examples of chiral [2]catenanes and [2]rotaxanes, and discusses where these molecules have been used in chemical applications such as chiral host-guest recognition and asymmetric catalysis.
Keywords: catalysis; catenanes; chirality; host-guest recognition; rotaxanes.
© 2018 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures






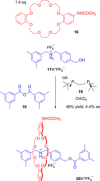
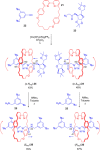
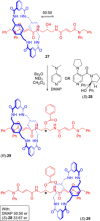
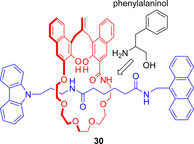

References
-
- Chirality in Supramolecular Assemblies: Causes and Consequences (Ed.: F. R. Keene), Wiley, Chichester, 2016.
-
- Berthod A., Anal. Chem. 2006, 78, 2093–2099. - PubMed
-
- Evans N. H., Beer P. D., Chem. Soc. Rev. 2014, 43, 4658–4683. - PubMed
-
- Xue M., Yang Y., Chi X., Yan X., Huang F., Chem. Rev. 2015, 115, 7398–7501. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

