Allelic Expression Imbalance in the Human Retinal Transcriptome and Potential Impact on Inherited Retinal Diseases
- PMID: 29053642
- PMCID: PMC5664133
- DOI: 10.3390/genes8100283
Allelic Expression Imbalance in the Human Retinal Transcriptome and Potential Impact on Inherited Retinal Diseases
Abstract
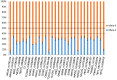
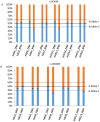
Inherited retinal diseases (IRDs) are often associated with variable clinical expressivity (VE) and incomplete penetrance (IP). Underlying mechanisms may include environmental, epigenetic, and genetic factors. Cis-acting expression quantitative trait loci (cis-eQTLs) can be implicated in the regulation of genes by favoring or hampering the expression of one allele over the other. Thus, the presence of such loci elicits allelic expression imbalance (AEI) that can be traced by massive parallel sequencing techniques. In this study, we performed an AEI analysis on RNA-sequencing (RNA-seq) data, from 52 healthy retina donors, that identified 194 imbalanced single nucleotide polymorphisms(SNPs) in 67 IRD genes. Focusing on SNPs displaying AEI at a frequency higher than 10%, we found evidence of AEI in several IRD genes regularly associated with IP and VE (BEST1, RP1, PROM1, and PRPH2). Based on these SNPs commonly undergoing AEI, we performed pyrosequencing in an independent sample set of 17 healthy retina donors in order to confirm our findings. Indeed, we were able to validate CDHR1, BEST1, and PROM1 to be subjected to cis-acting regulation. With this work, we aim to shed light on differentially expressed alleles in the human retina transcriptome that, in the context of autosomal dominant IRD cases, could help to explain IP or VE.
Keywords: Inherited retinal diseases; allelic expression imbalance; expressivity; penetrance; retina.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Analyzing allele specific RNA expression using mixture models.BMC Genomics. 2015 Aug 1;16(1):566. doi: 10.1186/s12864-015-1749-0. BMC Genomics. 2015. PMID: 26231172 Free PMC article.
-
Next-generation DNA sequencing-based assay for measuring allelic expression imbalance (AEI) of candidate neuropsychiatric disorder genes in human brain.BMC Genomics. 2011 Oct 20;12:518. doi: 10.1186/1471-2164-12-518. BMC Genomics. 2011. PMID: 22013986 Free PMC article.
-
Tryptophan hydroxylase 2 (TPH2) haplotypes predict levels of TPH2 mRNA expression in human pons.Mol Psychiatry. 2007 May;12(5):491-501. doi: 10.1038/sj.mp.4001923. Epub 2006 Dec 12. Mol Psychiatry. 2007. PMID: 17453063
-
Natural Selection on Exonic SNPs Shapes Allelic Expression Imbalance (AEI) Adaptability in Lung Cancer Progression.Front Genet. 2020 Jun 24;11:665. doi: 10.3389/fgene.2020.00665. eCollection 2020. Front Genet. 2020. PMID: 32670357 Free PMC article.
-
Searching for polymorphisms that affect gene expression and mRNA processing: example ABCB1 (MDR1).AAPS J. 2006 Aug 18;8(3):E515-20. doi: 10.1208/aapsj080361. AAPS J. 2006. PMID: 17025270 Free PMC article. Review.
Cited by
-
Genetic analyses of human fetal retinal pigment epithelium gene expression suggest ocular disease mechanisms.Commun Biol. 2019 May 20;2:186. doi: 10.1038/s42003-019-0430-6. eCollection 2019. Commun Biol. 2019. PMID: 31123710 Free PMC article.
-
An integrated transcriptional analysis of the developing human retina.Development. 2019 Jan 29;146(2):dev169474. doi: 10.1242/dev.169474. Development. 2019. PMID: 30696714 Free PMC article.
-
Patient derived stem cells for discovery and validation of novel pathogenic variants in inherited retinal disease.Prog Retin Eye Res. 2021 Jul;83:100918. doi: 10.1016/j.preteyeres.2020.100918. Epub 2020 Oct 29. Prog Retin Eye Res. 2021. PMID: 33130253 Free PMC article. Review.
-
Absence of Genotype/Phenotype Correlations Requires Molecular Diagnostic to Ascertain Stargardt and Stargardt-Like Swiss Patients.Genes (Basel). 2021 May 26;12(6):812. doi: 10.3390/genes12060812. Genes (Basel). 2021. PMID: 34073554 Free PMC article.
-
Variability in Gene Expression is Associated with Incomplete Penetrance in Inherited Eye Disorders.Genes (Basel). 2020 Feb 9;11(2):179. doi: 10.3390/genes11020179. Genes (Basel). 2020. PMID: 32050448 Free PMC article.
References
-
- RetNet - Retinal Information Network. [(accessed on 30 June 2017)]; Available online: https://sph.uth.edu/retnet/
-
- Bravo-Gil N., Méndez-Vidal C., Romero-Pérez L., González-del Pozo M., Rodríguez-de la Rúa E., Dopazo J., Borrego S., Antiñolo G. Improving the management of Inherited Retinal Dystrophies by targeted sequencing of a population-specific gene panel. Sci Rep. 2011;6:23910. doi: 10.1038/srep23910. - DOI - PMC - PubMed
-
- Louie C.M., Caridi G., Lopes V.S., Brancati F., Kispert A., Lancaster M.A., Schlossman A.M., Otto E.A., Leitges M., Gröne H.-J., et al. AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nat. Genet. 2011;42:175–180. doi: 10.1038/ng.519. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

