XX Disorder of Sex Development is associated with an insertion on chromosome 9 and downregulation of RSPO1 in dogs (Canis lupus familiaris)
- PMID: 29053721
- PMCID: PMC5650465
- DOI: 10.1371/journal.pone.0186331
XX Disorder of Sex Development is associated with an insertion on chromosome 9 and downregulation of RSPO1 in dogs (Canis lupus familiaris)
Abstract
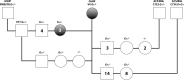
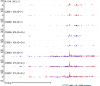
Remarkable progress has been achieved in understanding the mechanisms controlling sex determination, yet the cause for many Disorders of Sex Development (DSD) remains unknown. Of particular interest is a rare XX DSD subtype in which individuals are negative for SRY, the testis determining factor on the Y chromosome, yet develop testes or ovotestes, and both of these phenotypes occur in the same family. This is a naturally occurring disorder in humans (Homo sapiens) and dogs (C. familiaris). Phenotypes in the canine XX DSD model are strikingly similar to those of the human XX DSD subtype. The purposes of this study were to identify 1) a variant associated with XX DSD in the canine model and 2) gene expression alterations in canine embryonic gonads that could be informative to causation. Using a genome wide association study (GWAS) and whole genome sequencing (WGS), we identified a variant on C. familiaris autosome 9 (CFA9) that is associated with XX DSD in the canine model and in affected purebred dogs. This is the first marker identified for inherited canine XX DSD. It lies upstream of SOX9 within the canine ortholog for the human disorder, which resides on 17q24. Inheritance of this variant indicates that XX DSD is a complex trait in which breed genetic background affects penetrance. Furthermore, the homozygous variant genotype is associated with embryonic lethality in at least one breed. Our analysis of gene expression studies (RNA-seq and PRO-seq) in embryonic gonads at risk of XX DSD from the canine model identified significant RSPO1 downregulation in comparison to XX controls, without significant upregulation of SOX9 or other known testis pathway genes. Based on these data, a novel mechanism is proposed in which molecular lesions acting upstream of RSPO1 induce epigenomic gonadal mosaicism.
Conflict of interest statement
Figures




Similar articles
-
Association between polymorphisms in the SOX9 region and canine disorder of sex development (78,XX; SRY-negative) revisited in a multibreed case-control study.PLoS One. 2019 Jun 20;14(6):e0218565. doi: 10.1371/journal.pone.0218565. eCollection 2019. PLoS One. 2019. PMID: 31220175 Free PMC article.
-
Whole-genome de novo sequencing reveals genomic variants associated with differences of sex development in SRY negative pigs.Biol Sex Differ. 2024 Sep 2;15(1):68. doi: 10.1186/s13293-024-00644-w. Biol Sex Differ. 2024. PMID: 39223676 Free PMC article.
-
Deep sequencing of a candidate region harboring the SOX9 gene for the canine XX disorder of sex development.Anim Genet. 2017 Jun;48(3):330-337. doi: 10.1111/age.12538. Epub 2017 Jan 17. Anim Genet. 2017. PMID: 28094446
-
Testicular differentiation in 46,XX DSD: an overview of genetic causes.Front Endocrinol (Lausanne). 2024 Apr 24;15:1385901. doi: 10.3389/fendo.2024.1385901. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38721146 Free PMC article. Review.
-
Disorders of Sex Development with Testicular Differentiation in SRY-Negative 46,XX Individuals: Clinical and Genetic Aspects.Sex Dev. 2016;10(1):1-11. doi: 10.1159/000445088. Epub 2016 Apr 8. Sex Dev. 2016. PMID: 27055195 Review.
Cited by
-
Association between polymorphisms in the SOX9 region and canine disorder of sex development (78,XX; SRY-negative) revisited in a multibreed case-control study.PLoS One. 2019 Jun 20;14(6):e0218565. doi: 10.1371/journal.pone.0218565. eCollection 2019. PLoS One. 2019. PMID: 31220175 Free PMC article.
-
Genomic Sequencing to Detect Cross-Breeding Quality in Dogs: An Example Studying Disorders in Sexual Development.Int J Mol Sci. 2024 Oct 6;25(19):10763. doi: 10.3390/ijms251910763. Int J Mol Sci. 2024. PMID: 39409092 Free PMC article.
-
Genome sequencing of 2000 canids by the Dog10K consortium advances the understanding of demography, genome function and architecture.Genome Biol. 2023 Aug 15;24(1):187. doi: 10.1186/s13059-023-03023-7. Genome Biol. 2023. PMID: 37582787 Free PMC article.
-
An Integrated Database for Exploring Alternative Promoters in Animals.Sci Data. 2025 Feb 7;12(1):231. doi: 10.1038/s41597-025-04548-1. Sci Data. 2025. PMID: 39920194 Free PMC article.
-
Clinical and diagnostic approach of male pseudo hermaphroditism with os-clitoris in French bulldog: A case report.Vet Med Sci. 2022 May;8(3):953-958. doi: 10.1002/vms3.764. Epub 2022 Feb 13. Vet Med Sci. 2022. PMID: 35156328 Free PMC article.
References
-
- Eggers S, Sadedin S, van den Bergen JA, Robevska G, Ohnesorg T, Hewitt J, et al. Disorders of sex development: insights from targeted gene sequencing of a large international patient cohort. Genome Biol. 2016. November 29;17(1):243 doi: 10.1186/s13059-016-1105-y - DOI - PMC - PubMed
-
- Munger SC, Capel B. Sex and the circuitry: progress toward a systems‐level understanding of vertebrate sex determination. Wiley Interdiscip Rev Syst Biol Med. 2012. July 1;4(4):401–12. doi: 10.1002/wsbm.1172 - DOI - PMC - PubMed
-
- Eggers S, Ohnesorg T, Sinclair A. Genetic regulation of mammalian gonad development. Nat Rev Endocrinol. 2014. November 1;10(11):673–83. doi: 10.1038/nrendo.2014.163 - DOI - PubMed
-
- Bashamboo A, McElreavey K. Human sex-determination and disorders of sex-development (DSD). Semin Cell Dev Biol. 2015. September;45:77–83. doi: 10.1016/j.semcdb.2015.10.030 - DOI - PubMed
-
- Biason-Lauber A, Chaboissier MC. Ovarian development and disease: the known and the unexpected. Semin Cell Dev Biol. 2015. September;45:59–67. doi: 10.1016/j.semcdb.2015.10.021 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

