Concepts and Strategies in Retinal Gene Therapy
- PMID: 29053763
- PMCID: PMC5656413
- DOI: 10.1167/iovs.17-22978
Concepts and Strategies in Retinal Gene Therapy
Figures

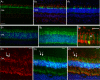

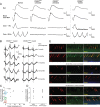

References
-
- Bramall AN, Wright AF, Jacobson SG, McInnes RR. . The genomic, biochemical, and cellular responses of the retina in inherited photoreceptor degenerations and prospects for the treatment of these disorders. Annu Rev Neurosci. 2010; 33: 441– 472. - PubMed
-
- Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. . Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010; 11: 273– 284. - PubMed
-
- Acland GM, Aguirre GD, Ray J,et al. . Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001; 28: 92– 95. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

