Clinicopathological correlations in behavioural variant frontotemporal dementia
- PMID: 29053860
- PMCID: PMC5841140
- DOI: 10.1093/brain/awx254
Clinicopathological correlations in behavioural variant frontotemporal dementia
Abstract
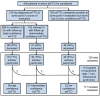
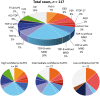
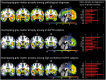
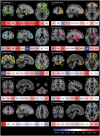
Accurately predicting the underlying neuropathological diagnosis in patients with behavioural variant frontotemporal dementia (bvFTD) poses a daunting challenge for clinicians but will be critical for the success of disease-modifying therapies. We sought to improve pathological prediction by exploring clinicopathological correlations in a large bvFTD cohort. Among 438 patients in whom bvFTD was either the top or an alternative possible clinical diagnosis, 117 had available autopsy data, including 98 with a primary pathological diagnosis of frontotemporal lobar degeneration (FTLD), 15 with Alzheimer's disease, and four with amyotrophic lateral sclerosis who lacked neurodegenerative disease-related pathology outside of the motor system. Patients with FTLD were distributed between FTLD-tau (34 patients: 10 corticobasal degeneration, nine progressive supranuclear palsy, eight Pick's disease, three frontotemporal dementia with parkinsonism associated with chromosome 17, three unclassifiable tauopathy, and one argyrophilic grain disease); FTLD-TDP (55 patients: nine type A including one with motor neuron disease, 27 type B including 21 with motor neuron disease, eight type C with right temporal lobe presentations, and 11 unclassifiable including eight with motor neuron disease), FTLD-FUS (eight patients), and one patient with FTLD-ubiquitin proteasome system positive inclusions (FTLD-UPS) that stained negatively for tau, TDP-43, and FUS. Alzheimer's disease was uncommon (6%) among patients whose only top diagnosis during follow-up was bvFTD. Seventy-nine per cent of FTLD-tau, 86% of FTLD-TDP, and 88% of FTLD-FUS met at least 'possible' bvFTD diagnostic criteria at first presentation. The frequency of the six core bvFTD diagnostic features was similar in FTLD-tau and FTLD-TDP, suggesting that these features alone cannot be used to separate patients by major molecular class. Voxel-based morphometry revealed that nearly all pathological subgroups and even individual patients share atrophy in anterior cingulate, frontoinsula, striatum, and amygdala, indicating that degeneration of these regions is intimately linked to the behavioural syndrome produced by these diverse aetiologies. In addition to these unifying features, symptom profiles also differed among pathological subtypes, suggesting distinct anatomical vulnerabilities and informing a clinician's prediction of pathological diagnosis. Data-driven classification into one of the 10 most common pathological diagnoses was most accurate (up to 60.2%) when using a combination of known predictive factors (genetic mutations, motor features, or striking atrophy patterns) and the results of a discriminant function analysis that incorporated clinical, neuroimaging, and neuropsychological data.
Keywords: Alzheimer’s disease; Pick’s disease; corticobasal degeneration; frontotemporal dementia; frontotemporal lobar degeneration.
© The Author (2017). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Alladi S, Xuereb J, Bak T, Nestor P, Knibb J, Patterson K, et al.Focal cortical presentations of Alzheimer's disease. Brain 2007; 130: 2636–45. - PubMed
-
- Chare L, Hodges JR, Leyton CE, McGinley C, Tan RH, Kril JJ, et al.New criteria for frontotemporal dementia syndromes: clinical and pathological diagnostic implications. J Neurol Neurosurg Psychiatry 2014; 85: 865–70. - PubMed
-
- Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, et al.Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol 2012; 11: 669–78. - PubMed
-
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994; 44: 2308–14. - PubMed
MeSH terms
Grants and funding
- K24 AG045333/AG/NIA NIH HHS/United States
- P01 AG017586/AG/NIA NIH HHS/United States
- K23 AG038357/AG/NIA NIH HHS/United States
- R01 AG038791/AG/NIA NIH HHS/United States
- R01 NS095257/NS/NINDS NIH HHS/United States
- U01 AG052943/AG/NIA NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- R01 NS050915/NS/NINDS NIH HHS/United States
- K01 AG055698/AG/NIA NIH HHS/United States
- K24 DC015544/DC/NIDCD NIH HHS/United States
- K23 AG045289/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- RF1 AG029577/AG/NIA NIH HHS/United States
- R01 AG052496/AG/NIA NIH HHS/United States
- P30 AG008051/AG/NIA NIH HHS/United States
- R01 AG032306/AG/NIA NIH HHS/United States
- K23 AG039414/AG/NIA NIH HHS/United States
- R01 AG022983/AG/NIA NIH HHS/United States
- U54 NS092089/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

