The C-terminal region of A-kinase anchor protein 350 (AKAP350A) enables formation of microtubule-nucleation centers and interacts with pericentriolar proteins
- PMID: 29054927
- PMCID: PMC5733579
- DOI: 10.1074/jbc.M117.806018
The C-terminal region of A-kinase anchor protein 350 (AKAP350A) enables formation of microtubule-nucleation centers and interacts with pericentriolar proteins
Abstract
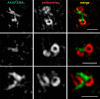
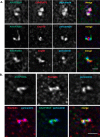
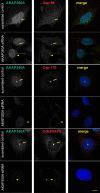
Microtubules in animal cells assemble (nucleate) from both the centrosome and the cis-Golgi cisternae. A-kinase anchor protein 350 kDa (AKAP350A, also called AKAP450/CG-NAP/AKAP9) is a large scaffolding protein located at both the centrosome and Golgi apparatus. Previous findings have suggested that AKAP350 is important for microtubule dynamics at both locations, but how this scaffolding protein assembles microtubule nucleation machinery is unclear. Here, we found that overexpression of the C-terminal third of AKAP350A, enhanced GFP-AKAP350A(2691-3907), induces the formation of multiple microtubule-nucleation centers (MTNCs). Nevertheless, these induced MTNCs lacked "true" centriole proteins, such as Cep135. Mapping analysis with AKAP350A truncations demonstrated that AKAP350A contains discrete regions responsible for promoting or inhibiting the formation of multiple MTNCs. Moreover, GFP-AKAP350A(2691-3907) recruited several pericentriolar proteins to MTNCs, including γ-tubulin, pericentrin, Cep68, Cep170, and Cdk5RAP2. Proteomic analysis indicated that Cdk5RAP2 and Cep170 both interact with the microtubule nucleation-promoting region of AKAP350A, whereas Cep68 interacts with the distal C-terminal AKAP350A region. Yeast two-hybrid assays established a direct interaction of Cep170 with AKAP350A. Super-resolution and deconvolution microscopy analyses were performed to define the association of AKAP350A with centrosomes, and these studies disclosed that AKAP350A spans the bridge between centrioles, co-localizing with rootletin and Cep68 in the linker region. siRNA-mediated depletion of AKAP350A caused displacement of both Cep68 and Cep170 from the centrosome. These results suggest that AKAP350A acts as a scaffold for factors involved in microtubule nucleation at the centrosome and coordinates the assembly of protein complexes associating with the intercentriolar bridge.
Keywords: A-kinase anchoring protein (AKAP); AKAP450; AKAP9; Cdk5RAP2; Cep170; Cep68; centrosome; microscopy; microtubule; protein kinase A (PKA).
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
A centrosomal scaffold shows some self-control.J Biol Chem. 2017 Dec 15;292(50):20410-20411. doi: 10.1074/jbc.H117.806018. J Biol Chem. 2017. PMID: 29247130 Free PMC article. Review.
-
Cep68 and Cep215 (Cdk5rap2) are required for centrosome cohesion.J Cell Sci. 2007 Dec 15;120(Pt 24):4321-31. doi: 10.1242/jcs.020248. Epub 2007 Nov 27. J Cell Sci. 2007. PMID: 18042621
-
Centrosome- and Golgi-Localized Protein Kinase N-Associated Protein Serves As a Docking Platform for Protein Kinase A Signaling and Microtubule Nucleation in Migrating T-Cells.Front Immunol. 2018 Mar 1;9:397. doi: 10.3389/fimmu.2018.00397. eCollection 2018. Front Immunol. 2018. PMID: 29545805 Free PMC article.
-
The DNA replication protein Cdc6 inhibits the microtubule-organizing activity of the centrosome.J Biol Chem. 2017 Sep 29;292(39):16267-16276. doi: 10.1074/jbc.M116.763680. Epub 2017 Aug 21. J Biol Chem. 2017. PMID: 28827311 Free PMC article.
-
The centrosome-Golgi apparatus nexus.Philos Trans R Soc Lond B Biol Sci. 2014 Sep 5;369(1650):20130462. doi: 10.1098/rstb.2013.0462. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25047616 Free PMC article. Review.
Cited by
-
A centrosomal scaffold shows some self-control.J Biol Chem. 2017 Dec 15;292(50):20410-20411. doi: 10.1074/jbc.H117.806018. J Biol Chem. 2017. PMID: 29247130 Free PMC article. Review.
-
FBF1 deficiency promotes beiging and healthy expansion of white adipose tissue.Cell Rep. 2021 Aug 3;36(5):109481. doi: 10.1016/j.celrep.2021.109481. Cell Rep. 2021. PMID: 34348145 Free PMC article.
-
Spatial and proteomic profiling reveals centrosome-independent features of centriolar satellites.EMBO J. 2019 Jul 15;38(14):e101109. doi: 10.15252/embj.2018101109. Epub 2019 Jun 3. EMBO J. 2019. PMID: 31304627 Free PMC article.
-
The TBC1D31/praja2 complex controls primary ciliogenesis through PKA-directed OFD1 ubiquitylation.EMBO J. 2021 May 17;40(10):e106503. doi: 10.15252/embj.2020106503. Epub 2021 May 2. EMBO J. 2021. PMID: 33934390 Free PMC article.
-
The E3 ligase Poe promotes Pericentrin degradation.Mol Biol Cell. 2023 Aug 1;34(9):br15. doi: 10.1091/mbc.E22-11-0534. Epub 2023 Jun 21. Mol Biol Cell. 2023. PMID: 37342879 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

