The type II transmembrane serine protease matriptase cleaves the amyloid precursor protein and reduces its processing to β-amyloid peptide
- PMID: 29054928
- PMCID: PMC5733603
- DOI: 10.1074/jbc.M117.792911
The type II transmembrane serine protease matriptase cleaves the amyloid precursor protein and reduces its processing to β-amyloid peptide
Abstract
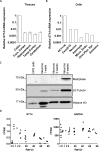
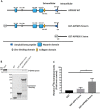
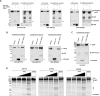
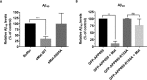
Recent studies have reported that many proteases, besides the canonical α-, β-, and γ-secretases, cleave the amyloid precursor protein (APP) and modulate β-amyloid (Aβ) peptide production. Moreover, specific APP isoforms contain Kunitz protease-inhibitory domains, which regulate the proteolytic activity of serine proteases. This prompted us to investigate the role of matriptase, a member of the type II transmembrane serine protease family, in APP processing. Using quantitative RT-PCR, we detected matriptase mRNA in several regions of the human brain with an enrichment in neurons. RNA sequencing data of human dorsolateral prefrontal cortex revealed relatively high levels of matriptase RNA in young individuals, whereas lower levels were detected in older individuals. We further demonstrate that matriptase and APP directly interact with each other and that matriptase cleaves APP at a specific arginine residue (Arg-102) both in vitro and in cells. Site-directed (Arg-to-Ala) mutagenesis of this cleavage site abolished matriptase-mediated APP processing. Moreover, we observed that a soluble, shed matriptase form cleaves endogenous APP in SH-SY5Y cells and that this cleavage significantly reduces APP processing to Aβ40. In summary, this study identifies matriptase as an APP-cleaving enzyme, an activity that could have important consequences for the abundance of Aβ and in Alzheimer's disease pathology.
Keywords: Alzheimer disease; ST14; amyloid precursor protein (APP); amyloid-β (Aβ); brain; enzyme processing; extracellular matrix protein; matriptase; serine protease.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Chiang P. K., Lam M. A., and Luo Y. (2008) The many faces of amyloid β in Alzheimer's disease. Curr. Mol. Med. 8, 580–584 - PubMed
-
- Arai H., Lee V. M., Messinger M. L., Greenberg B. D., Lowery D. E., and Trojanowski J. Q. (1991) Expression patterns of β-amyloid precursor protein (β-APP) in neural and nonneural human tissues from Alzheimer's disease and control subjects. Ann. Neurol. 30, 686–693 - PubMed
-
- Kitaguchi N., Takahashi Y., Tokushima Y., Shiojiri S., and Ito H. (1988) Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitory activity. Nature 331, 530–532 - PubMed
-
- Preece P., Virley D. J., Costandi M., Coombes R., Moss S. J., Mudge A. W., Jazin E., and Cairns N. J. (2004) Amyloid precursor protein mRNA levels in Alzheimer's disease brain. Brain Res. Mol. Brain Res. 122, 1–9 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

