Pivotal neuroinflammatory and therapeutic role of high mobility group box 1 in ischemic stroke
- PMID: 29054968
- PMCID: PMC5715129
- DOI: 10.1042/BSR20171104
Pivotal neuroinflammatory and therapeutic role of high mobility group box 1 in ischemic stroke
Abstract
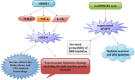
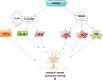
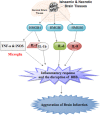
Stroke is a major cause of mortality and disability worldwide. Stroke is a frequent and severe neurovascular disorder. The main cause of stroke is atherosclerosis, and the most common risk factor for atherosclerosis is hypertension. Therefore, prevention and treatment of stroke are crucial issues in humans. High mobility group box 1 (HMGB1) is non-histone nuclear protein that is currently one of the crucial proinflammatory alarmins in ischemic stroke (IS). It is instantly released from necrotic cells in the ischemic core and activates an early inflammatory response. HMGB1 may signal via its putative receptors, such as receptor for advanced glycation end products (RAGE), toll-like receptors (TLRs) as well as matrix metalloproteinase (MMP) enzymes during IS. These receptors are expressed in brain cells. Additionally, brain-released HMGB1 can be redox modified in the circulation and activate peripheral immune cells. The role of HMGB1 may be more complex. HMGB1 possesses beneficial actions, such as endothelial activation, enhancement of neurite outgrowth, and neuronal survival. HMGB1 may also provide a novel link for brain-immune communication leading to post-stroke immunomodulation. Therefore, HMGB1 is new promising therapeutic intervention aimed at promoting neurovascular repair and remodeling after stroke. In this review, we look at the mechanisms of secretion of HMGB1, the role of receptors, MMP enzymes, hypoglycemia, atherosclerosis, edema, angiogenesis as well as neuroimmunological reactions and post-ischemic brain recovery in IS. We also outline therapeutic roles of HMGB1 in IS.
Keywords: HMGB1; Hypoglycaemia; Ischemic stroke; angiogenesis; atherosclerosis; oedema.
© 2017 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
References
-
- Singh V., Roth S., Veltkamp R. and Liesz A. (2016) HMGB1 as a key mediator of immune mechanisms in ischemic stroke. Antioxid. Redox Signal. 24, 635–651 - PubMed
-
- Kikuchi K., Tancharoen S., Ito T., Morimoto-Yamashita Y., Miura N., Kawahara K. et al. (2013) Potential of the angiotensin receptor blockers (ARBs) telmisartan, irbesartan, and candesartan for inhibiting the HMGB1/RAGE axis in prevention and acute treatment of stroke. Int. J. Mol. Sci. 14, 18899–18924 - PMC - PubMed
-
- Demaerschalk B.M., Hwang H.M. and Leung G. (2010) US cost burden of ischemic stroke: a systematic literature review. Am. J. Manag. Care 16, 525–533 - PubMed
-
- Furie K.L., Kasner S.E., Adams R.J., Albers G.W., Bush R.L., Fagan S.C. et al. (2011) Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42, 227–276 - PubMed
-
- Richard S.A., Xiang L.-H., Yun J.-X., Shanshan Z., Jiang Y.-Y, Wang J. et al. (2017) Carcinogenic and therapeutic role of high-mobility group box 1 in cancer: is it a cancer facilitator, a cancer inhibitor or both? World Cancer Res. J. 4, e919
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

