Amyloid β synaptotoxicity is Wnt-PCP dependent and blocked by fasudil
- PMID: 29055813
- PMCID: PMC5869054
- DOI: 10.1016/j.jalz.2017.09.008
Amyloid β synaptotoxicity is Wnt-PCP dependent and blocked by fasudil
Abstract
Introduction: Synapse loss is the structural correlate of the cognitive decline indicative of dementia. In the brains of Alzheimer's disease sufferers, amyloid β (Aβ) peptides aggregate to form senile plaques but as soluble peptides are toxic to synapses. We previously demonstrated that Aβ induces Dickkopf-1 (Dkk1), which in turn activates the Wnt-planar cell polarity (Wnt-PCP) pathway to drive tau pathology and neuronal death.
Methods: We compared the effects of Aβ and of Dkk1 on synapse morphology and memory impairment while inhibiting or silencing key elements of the Wnt-PCP pathway.
Results: We demonstrate that Aβ synaptotoxicity is also Dkk1 and Wnt-PCP dependent, mediated by the arm of Wnt-PCP regulating actin cytoskeletal dynamics via Daam1, RhoA and ROCK, and can be blocked by the drug fasudil.
Discussion: Our data add to the importance of aberrant Wnt signaling in Alzheimer's disease neuropathology and indicate that fasudil could be repurposed as a treatment for the disease.
Keywords: Alzheimer's disease; Amyloid; DAAM1; Dickkopf-1; Fasudil; Planar cell polarity; ROCK; Synapse; Synaptotoxicity; Wnt.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
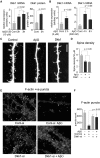
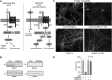
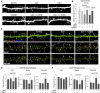


References
-
- Pozueta J., Lefort R., Shelanski M.L. Synaptic changes in Alzheimer's disease and its models. Neuroscience. 2013;251:51–65. - PubMed
-
- Bafico A., Liu G., Yaniv A., Gazit A., Aaronson S.A. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–686. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

