An Open-Label Randomized Multicenter Study Assessing the Noninferiority of a Caffeine-Based Topical Liquid 0.2% versus Minoxidil 5% Solution in Male Androgenetic Alopecia
- PMID: 29055953
- PMCID: PMC5804833
- DOI: 10.1159/000481141
An Open-Label Randomized Multicenter Study Assessing the Noninferiority of a Caffeine-Based Topical Liquid 0.2% versus Minoxidil 5% Solution in Male Androgenetic Alopecia
Abstract
Background: Androgenetic alopecia is a condition with a high prevalence worldwide and affects both males and females. Currently, only 2 approved treatments exist: finasteride (males only) and minoxidil 2 or 5% solution (males and females).
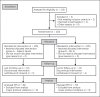
Methods: We conducted a randomized, open-label, multicenter noninferiority study to determine whether a caffeine-based 0.2% topical liquid would be no less effective than minoxidil 5% solution in males (n = 210) with androgenetic alopecia. The primary end point was the percentage change in the proportion of anagen hairs from baseline to 6 months using a frontal and occipital trichogram.
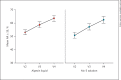
Results: At 6 months, the group of the 5% minoxidil solution showed a mean improvement in anagen ratio of the trichogram of 11.68%, and the group of the 0.2% caffeine solution had an anagen improvement of 10.59%. The difference of mean values between both groups was 1.09%. The statistical analysis was performed and reported in accordance with the CONSORT Guidelines 2010 for reporting of noninferiority and equivalence randomized trials.
Conclusion: A caffeine-based topical liquid should be considered as not inferior to minoxidil 5% solution in men with androgenetic alopecia.
Keywords: Anagen hairs; Androgenetic alopecia; Caffeine-based topical liquid; Frontal trichogram; Occipital trichogram.
© 2017 The Author(s) Published by S. Karger AG, Basel.
Figures
References
-
- Blumeyer A, Tosti A, Messenger A, Reygagne P, Del Marmol V, Spuls PI, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. 2011;9((suppl 6)):S1–S57. - PubMed
-
- Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. - PubMed
-
- Torres F. Androgenetic, diffuse and senescent alopecia in men: practical evaluation and management. Curr Probl Dermatol. 2015;47:33–44. - PubMed
-
- US Food and Drug Administration Drug facts - minoxidil 2% solution. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformat... (accessed March 1, 2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical